Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each
Question:
Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the followingacylbenzenes:
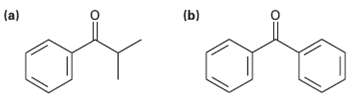
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Strategy To identify the carboxylic acid ...View the full answer

Answered By

Hafeeza Shaik
I possess a strong track record in improving test scores and teaching effectively.I have ability to be a team player and resolve problems and conflicts professionally. Have an ability to communicate complex information into a simple and entertaining manner.Looking to contribute my knowledge and skills in a platform like solutioninn that offers a genuine opportunity for career progression.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify ratios and expected relationships that might be used when performing preliminary analytical procedures related to longlived assets.
-
Identify two audit approaches that might be used to gain assurance about the correctness of perpetual inventory records.
-
Identify several tools that might be used as aids in the human resource planning process.
-
What is step-down allocation? What are some criticisms of this allocation method?
-
Brown Corporation (a calendar year taxpayer) provides you with the following information. Taxable income.............................................................................$2,600,000...
-
What sorts of restrictions or guidelines should firms place on the use of social networks by employees? Are these sites a threat to security? Can they tarnish a firms reputation? If so, how? Can they...
-
Claire Fitch is planning to begin an individual retirement program in which she will invest $1,500 at the end of each year. Fitch plans to retire after making 30 annual investments in the program...
-
At the beginning of a fiscal year, Alexander Company buys a machine for $ 48,000. The machine has an estimated life of five years and an estimated salvage value of $ 4,000. Required Using the...
-
5.4] (6-37 Weighted-Average Method Weatherly Lumber Company processes wood pulp for manufactur ing various paper products. The company employs a process costing system for its manufacturing...
-
What is output of the following program? #include using namespace std; int x = 19; int main () { int x = 21; { int x = 41; cout <
-
What is the major mono-substitution product from the Friedel-Crafts reaction of benzene with 1-chloro-2-methylpropane in the presence of AlCl3?
-
Write resonance structures for nitrobenzene to show the electron-withdrawing resonance effect of the nitro group.
-
The board of directors of Zeta Health Spa authorizes the issuance of \($500,000\) of 8%, 10-year bonds payable. The semiannual interest dates are May 31 and November 30. The bonds are issued on July...
-
The table below shows the population of Mozambique between 1960 and 2010. This data can be modelled using an exponential function of the form P = ab t , where t is the time in years since 1960 and a...
-
Southco is a medium-sized American-owned global manufacturer of access hardware solutions, such as latches and hinges, used for applications in the aircraft, railway, computer and automotive...
-
To what extent do staffing processes at the Dionysos reflect the strategic approach to recruitment and selection encapsulated by the conceptual framework and model depicted in Key Concepts 8.4 and...
-
Im an accounting major, not an operations expert, yelled just-promoted Bob Barthrow, the executive vice president of the Midwest Frequent Flyer Call Center, during a senior-level management meeting....
-
The Hudson Jewelers case study can be found in Appendix C. Chapter 14 Case Question for Discussion: 1.Customer demand (weekly visits) at Hudson Jewelers is highly seasonal, as shown in the worksheet...
-
Which four statements are least appropriate? There are several hot tips for organizations when going to the market for competitive bid including the following: a. Make a good business case for the...
-
Activator rod AB exerts on crank BCD a force P directed along line AB. Knowing that P must have a 100-N component perpendicular to arm BC of the crank, determine (a) The magnitude of the force P, (b)...
-
a. Show for the SoaveRedlich-Kwong equation of state (Eq. 6.4-1) that b. Show that the critical compressibility of the SoaveRedlich-Kwong equation of state is 1/3. a(T) = 0.427 48- -a (T) RT Pc RTC...
-
Heating ethanol with sulfuric acid is one method used for the preparation of diethyl ether. Show all of the steps in the mechanism for this reaction: H,SO4 2 CH;CH,OH CH;CH,-0-CH,CH, + H2O
-
When an aqueous solution of (R)-2-butanol is treated with a catalytic amount of sulfuric acid, slow racemization of the alcohol occurs. Show all of the steps in the mechanism for this process.
-
Show all of the steps in the mechanism and explain the stereo chemistry for this reaction: Br , Br + . + C-C CH3 . CH3 .
-
Green Lawn Company sells garden supplies. Management is planning its cash needs for the second quarter. The following information has been assembled to assist in preparing a cash budget for the...
-
eBook Question Content Area Comparison of Methods of Allocation Duweynie Pottery, Inc., is divided into two operating divisions: Pottery and Retail. The company allocates Power and General Factory...
-
TYBALT CONSTRUCTION Income Statement For Year Ended December 31 TYBALT CONSTRUCTION Income Statement For Year Ended December 31

Study smarter with the SolutionInn App


