Identify the chirality centers in the following molecules (yellow-green = Cl, pale yellow =F): (b) (a) Threose
Question:
Identify the chirality centers in the following molecules (yellow-green = Cl, pale yellow =F):
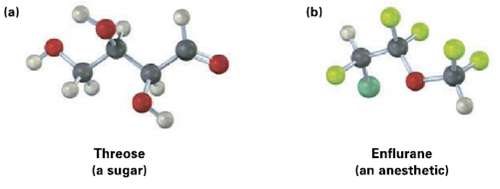
Transcribed Image Text:
(b) (a) Threose (a sugar) Enflurane (an anesthetic)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
a H H H ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify the chirality centers in the following molecules. Build molecular models if you needhelp. (b) (c) CH30. CH2CH2CH3 C (a) - Coniine (poison hemlock) N-CH3 Menthol (flavoring agent)...
-
Assign R or S stereochemistry to the chirality centers in the following Newmanprojections: CI (a) (b) C "CH . "
-
Assign R or S configurations to the chirality centers in the followingmolecules: CI H la) (c) (b) H
-
Outline suggestions to make observation a useful, reliable requirements elicitation technique.
-
Discuss why sound travels faster in moist air. (Note that at the same temperature, water vapor molecules have the same average kinetic energy as the heavier nitrogen and oxygen molecules in the air.)...
-
Until recently, American cars exported to Japan had driver controls on the left side (as in the United States), even though Japanese cars sold in Japan have driver controls on the right side, because...
-
Using the PewSocialMedia dataset to run a set of nested regression models to investigate the following hypotheses: 1. Having knowledge and interest about the U.S. surveillance program (as measured by...
-
Vance Asbestos Removal Company removes potentially toxic asbestos insulation and related products from buildings. The companys estimator has been involved in a long-simmering dispute with the on-site...
-
Ratio of liabilities to stockholders equity and times interest earned The following data were taken from the financial statements of Hunter Inc. for December 3 1 of two recent years: Line Item...
-
(a) Draw resonance structures for the carbocation that could be formed from (E)-2-butenyl trifluoromethanesulfonate. (b) One of the resonance structures for this carbocation should be a more...
-
Alanine, an amino add found in proteins, is chiral. Draw the two enantiorners of ala- nine using the standard convention of solid, wedged, and dashedlines. NH2 Alanine CHO2H
-
Is cocaine (Worked Example 9.2) dextrorotatory or levorotatory?
-
Based on your observations, describe another possible kind of knowledge that needs to be discovered by data mining methods but has not been listed in this chapter. Does it require a mining...
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Given the following HTML, write a simple bit of JavaScript code that will DELETE ALL OF THE TAGS ON THE PAGE. Quiz I'm a Heading I'm a paragraph I'm special I'm also a paragraph Footer! HINT: You'll...
-
Your company has been quite successful in sending employees on international assignments. As the HR Manager responsible for selecting such employees, present a report to the management of your...
-
You will be looking at a particular market in the economy. I will assign the market to you arbitrarily. Please look for at the end of this document to identify which market you will be responsible...
-
Describe the chief strengths of both Excel and Access. Summarize the primary purposes of Excel and Access. Give an example of why you might want to process Access data in Excel and another example of...
-
Portal Manufacturing has total fixed costs of $520,000. A unit of product sells for $15 and variable costs per unit are $11. a). Prepare a contribution margin income statement showing predicted net...
-
Methanol (CH 3 OH) can be synthesized by the reaction: What volume (in liters) of hydrogen gas, at a temperature of 355 K and a pressure of 738 mmHg, is needed to synthesize 35.7 g of methanol? CO(g)...
-
Predict the product when each of the following compounds reacts with one equivalent of lithium dimethylcuprate, followed by protonolysis. Explain. CH,(CH,),0-C(CH,),C-CI
-
Outline two methods for the preparation of 5-methylhexanoic acid from 1-bromo-4-methylpentane.
-
One interesting process for making nylon-6,6 demonstrates the potential of using biomass as an industrial starting material. The raw material for this process, outlined in the following reaction, is...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


