In the iodoform reaction, a triiodomethyl ketone reacts with aqueous NaOH to yield a carboxylate ion and
Question:
In the iodoform reaction, a triiodomethyl ketone reacts with aqueous NaOH to yield a carboxylate ion and iodoform (triiodomethane). Propose a mechanism for thisreaction.
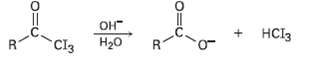
Transcribed Image Text:
OH H20 Cl3 HCI3 R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
This is a nucleophilic acyl substitution reaction who...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
Propose a mechanism that shows why p-chlorotoluene reacts with sodium hydroxide at 350 C to give a mixture of p-cresol and m-cresol.
-
Propose a mechanism for the reaction of acetyl chloride with phenylmagnesium bromide to give 1,1-diphenylethanol. OH (1) ether solvent CH3-C CI 2 (2) H 0 acetyl chloride phenylmagnesium bromide...
-
Consider the following function. def f(x): a = [] while x > 0: a.append(x) f(x-1) A new object of type list is created for each recursive invocation of f. True or False
-
Explain how blogs and social media networks can be used by businesses and report writers.
-
Suppose you have the opportunity to make an investment in a real estate venture that expects to pay investors $750 at the end of each month for the next eight years. You believe that a reasonable...
-
44. Beaver Corporation reported taxable income of $500,000 from operations this year. During the year, the company made a distribution of land to its sole shareholder, Eugenia VanDam. The lands fair...
-
Your assignment is to assist Jim in the preparation of a merchandise budget plan. First, fill out the merchandise budget form for six months. You may use either the form accompanying this case or the...
-
Comment on Peytons recent financial performances Quantify the impact of the accounting policy, and estimate changes being proposed by McNeilly on FY 2015s earnings relative to the figures that would...
-
A fabrication shop makes two types of valve actuators for explosive environments call them A and B. This actuator requires special manufacturing processes so there is one production line dedicated...
-
Epoxy adhesives are prepared in two steps. SN2 reaction of the disodium salt of bisphenol A with epichiorohydrin forms a ?prepolymer,? which is then ?cured? by treatment with a triamine such as H 2...
-
Draw structures for the enol tautomers of the following compounds: (a) Cyclopentanone (b) Methyl thioacetate (c) Ethyl acetate (d) Propanal (e) Acetic acid (f) Phenyl acetone
-
Define a change in estimate and provide an illustration. When is a change in accounting estimate effected by a change in accounting principle?
-
X 18. State the amplitude and period of: y = -4cos Graph one cycle of the function. 4 1 19. State the amplitude and period of: y = -sin(4x) Graph one cycle of the function. 4
-
Explain ways in which an organisation may overcome security vulnerabilities and issues?
-
A nonpipelined system takes 300ns to process a task. The same task can be processed in a 4-stage pipeline with a clock cycle of 50ns. Determine the speedup ratio of the pipeline for 400 tasks. What...
-
Within an orthodontic practice that I work in, insufficient patient care and poor time management are the most significant issues in the office. Beginning with the receptionists, scheduling...
-
How has the decision been improved with more of a focus on financial information? Why would it have been a better decision? How could you have included more financial information and where might it...
-
What are the major sources of competitive advantages of an organization that can be effectively developed to support a cost leadership strategy for competing in the market? AppendixLO1
-
The Smiths buy a house. They borrow 80 percent of the purchase price from the local ABC Savings and Loan. Before they make their first payment, ABC transfers the right to receive mortgage payments to...
-
Explain the difference between hybrid atomic orbitals in valence bond theory and LCAO molecular orbitals in MO theory.
-
Explain why The methyl group in the following compound has an unusual chemical shift of (- 1.61), about 4 ppm lower than the chemical shift of a typical allylic methyl group. : Na sodium salt of...
-
Explain why The methyl group in the following compound has an unusual chemical shift of (- 1.61), about 4 ppm lower than the chemical shift of a typical allylic methyl group. : Na sodium salt of...
-
Within each set, which compound should show NMR absorptions with the greater chemical shifts? Explain your choices. (1) (2)
-
Use the following information: \ table [ [ Country , \ table [ [ Consumer Prices ] ] , Interest Rates,Current Units ( per US$ ) ] , [ Forecast , 3 - month, 1 - yx Covt Bond,, ] , [ 2 0 2 4 e ,...
-
Year-to-date, Yum Brands had earned a 3.70 percent return. During the same time period, Raytheon earned 4.58 percent and Coca-Cola earned 0.53 percent. If you have a portfolio made up of 40 percent...
-
Rate of Return If State Occurs State of Probability of Economy State of Economy Stock A Stock B Stock C Boom .15 .31 .41 .21 Good .60 .16 .12 .10 Poor .20 .03 .06 .04 Bust .05 .11 .16 .08 a. Your...

Study smarter with the SolutionInn App


