Name the following cycloalkenes: .CH CH -CH (b) -CCH32 (a) (c) CH
Question:
Name the following cycloalkenes:
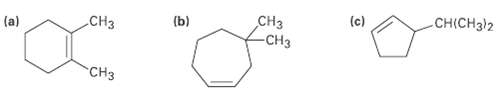
Transcribed Image Text:
.CHз CHз -CHз (b) -CНCH32 (a) (c) "CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
5 CH3 CH3 12Dimethyl ...View the full answer

Answered By

Rohail Amjad
Experienced Finance Guru have a full grip on various sectors, i.e Media, Insurance, Automobile, Rice and other Financial Services.
Have also served in Business Development Department as a Data Anlayst
4.70+
32+ Reviews
83+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Rank the following cycloalkenes in order of increasing stability.
-
Four different cycloalkenes will all yield methylcyclopentane when subjected to catalytic hydrogenation. What are their structures? Show the reactions.
-
Name Brand, Inc., is a small business. Twelve members of a single family own all of its stock. Or dinarily, corporate income is taxed at the corporate and shareholder levels. How can Name Brand avoid...
-
A 0.250-H inductor carries a time-varying current given by the expression i = (l24 mA) cos [(240 /s) t]. (a) Find an expression for the induced emf as a function of time. Graph the current and...
-
Why are both a task emphasis and a relationship emphasis often necessary to get a group through a crisis such as a hurricane having destroyed a company facility?
-
A uniform volume charge density of 80 C/m 3 is present throughout the region 8 mm < r < 10 mm. Let = 0 for 0 < r < 8 mm. (a) Find the total charge inside the spherical surface r = 10 mm. (b) Find...
-
Why might railroads have such low total assets turnovers and food wholesalers and grocery stores such high turnovers? AppendixLO1
-
On the basis of the following data, the general manager of Sole Mates Inc. decided to discontinue Children's Shoes because it reduced income from operations by $28,000. What is the flaw in...
-
The net income reported on the income statement for the current year was $73,600. Depreciation recorded on store equipment for the year amounted to $27,400. Balances of the current asset and current...
-
The drag force exerted on a car by air depends on a dimensionless drag coefficient, the density of air, the car velocity, and the frontal area of the car. That is, F D = F D (C drag , A front , , V)....
-
Draw structures corresponding to the following IUPAC names: (a) 2-Methyl-1, 5-hexadiene (b) 3-Ethyl-2, 2-dimethyl-3-heptene (c) 2, 3, 3-Trimethyl-1, 4, 6-octatriene (d) 3, 4-Diisopropyl-2,...
-
Which of the following compounds can exist as pairs of cisTrans isomers? Draw each cisTrans pair, and indicate the geometry of each isomer. (a) CH3CH = CH2 (b) (CH3)2C = CHCH3 (c) CH3CH2CH = CHCH3...
-
(FIFO cost per EUP) The following information has been gathered from the records of Snack-On Foods for July 1997. The firm makes a variety of snacks; the information presented here is for a peanut...
-
1. What are the threats being faced by Indian General Insurance Ltd. (IGIL)? 2. What are its traditional strengths? What 'business definitions' should it follow while capitalizing on its traditional...
-
You go to discuss the incident and the client's claims with your supervisor. As you retell the incident, it is clear that your supervisor is not comfortable. You ask your supervisor for advice on the...
-
Case Study Two: Rawlings Rawlings is an American sports equipment manufacturing company based in Town and Country, Missouri, and founded in 1887. Rawings specializes in baseball equipment and...
-
The discussion is for Administrating organizational change course. (we should write 300 words) Discussion question is: Refer to table 6.4 in your book. Think of a time when you were introduced to...
-
Content: Identify at least two resources for each of the four critical sections in the course project: Strategic Planning, Healthcare Reimbursement, Revenue Cycle Process, and Reimbursement...
-
Will you let me know if you think of anything else?
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
The phase diagram for sulfur is shown here. The rhombic and monoclinic states are two solid states with different structures. a. Below what pressure does solid sulfur sublime? b. Which of the two...
-
Organic compounds can contain many different functional groups. Identify the functional groups (aside from the alkane carbons) present in acebutolol (Fig. P2.47), a drug that blocks a certain part of...
-
Organic compounds can contain many different functional groups. Identify the functional groups (aside from the alkane carbons) present in acebutolol (Fig. P2.47), a drug that blocks a certain part of...
-
(a) Draw the structure of two other amides with the formula C4H9NO that do not contain isopropyl groups. (b) Could a compound with the formula C4H9NO contain a nitrile functional group? Explain.
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


