Primary amines react with esters to yield amides: RCO2R' + R''NH2 ? RCONHR'' + R'OH. Propose a
Question:
Primary amines react with esters to yield amides: RCO2R' + R''NH2 ? RCONHR'' + R'OH. Propose a mechanism for the following reaction of an ?, ? un-saturated ester.
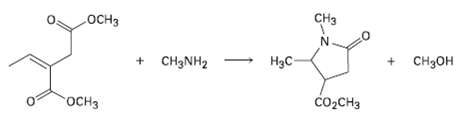
Transcribed Image Text:
CHз .ОCHЗ + CH3NH2 H3C- + CH3OH госнз CO2CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
CH3NH H3C CHO CH3O CHOH HC HC loss of methanol CH3NH HC conjugate addition of methy...View the full answer

Answered By

Sandhya Sharma
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
119+ Reviews
214+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the following reaction: CH CH, C-CH, CH2OH Cl CH3
-
Propose a mechanism for the following reaction: CI Alcl
-
Propose a mechanism for the following reaction. (The rate of the reaction is much slower if the nitrogen atom is replaced by CH.) CI - H2o CH3 N CI
-
Read the case study about Joy Jowie Inc and write a detailed paper about it
-
1. As Social Enterprises try to get larger, what unique challenges do they face? 2. What are some of the drawbacks associated with partnering with governments or big businesses? If you ran an SE,...
-
On average, a textbook author makes two wordprocessing errors per page on the first draft of her textbook. What is the probability that on the next page she will make (a) 4 or more errors? (b) No...
-
How is inflation dealt with when interest rates are determined by investors in the financial markets? AppendixLO1
-
On April 1, Holly Dahl established Holiday Travel Agency. The following transactions were completed during the month. 1. Stockholders invested $10,000 cash in the business in exchange for common...
-
Apocalyptica Corporation is expected to pay the following dividends over the next four years: $6.30, $17.30, $22.30, and $4.10. Afterward, the company pledges to maintain a constant 5.75 percent...
-
The Carrollton Buffet offers an all-you-can-eat buffet meal for $30 per person. The restaurant employs ten salaried employees. Rent for the building, employee salaries, and other fixed costs for the...
-
Propose structures for ketones or aldehydes that have the following 1 H NMR spectra: (a) C 10 H 12 O ? ??IR: 1710 cm ?1 ? (b) C 6 H 12 O 3 ? ? ?IR: 1715 cm ?1 ? (c) C 4 H 6 O ? ? ? ?IR: 1690 cm ?1 ?...
-
When crystals of pure ?-glucose are dissolved in water, isomerization slowly occurs to produce ?-glucose. Propose a mechanism for the isomerization. CH2 CH2 - - B-Glucose a-Glucose
-
Statement of Financial Position If a firm is to cut costs as a result of falling revenues, how would this appear in the statement of financial position? Explain.
-
1. How will you check if a class is a child of another class? 2. What is init method in python?
-
1. What are lists and tuples? What is the key difference between the two? 2. What is Scope in Python?
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
Q.1 If denotes increasing order of intensity, then the meaning of the words [talk shout scream] is analogous to [please pander]. Which one of the given options is appropriate to fill the blank? (A)...
-
Using this list of corporate values and Exhibit 3-5, describe Zappos organizational culture. In which areas would you say that Zappos culture is very high (or typical)? Explain.
-
Let (X. A. p) be a measure space. Show that for any A,B A, we have the equality: (AUB)+(An B) = (A) + (B).
-
Argon has a normal boiling point of 87.2 K and a melting point (at 1 atm) of 84.1 K. Its critical temperature is 150.8 K, and its critical pressure is 48.3 atm. It has a triple point at 83.7 K and...
-
Para-nitrosation of N,N-dimethylaniline (C-nitrosation) is believed to take place through an electrophilic attack by N+O ions. (a) Show how N+O ions might be formed in an aqueous solution of NaNO 2...
-
If we examine Table 21.1, we find that the methylphenols (cresols) are less acidic than phenol itself. For example, This behavior is characteristic of phenols bearing electron-releasing groups....
-
When o-chlorotoluene is subjected to the conditions used in the Dow process (i.e., aqueous NaOH at 350oC at high pressure), the products of the reaction are o-cresol and m-cresol. What does this...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases

Study smarter with the SolutionInn App


