Propose structures for ketones or aldehydes that have the following 1 H NMR spectra: (a) C 10
Question:
Propose structures for ketones or aldehydes that have the following 1H NMR spectra:
(a) C10H12O ? ??IR: 1710 cm?1?
(b) C6H12O3? ? ?IR: 1715 cm?1?
(c) C4H6O ? ? ? ?IR: 1690 cm?1?
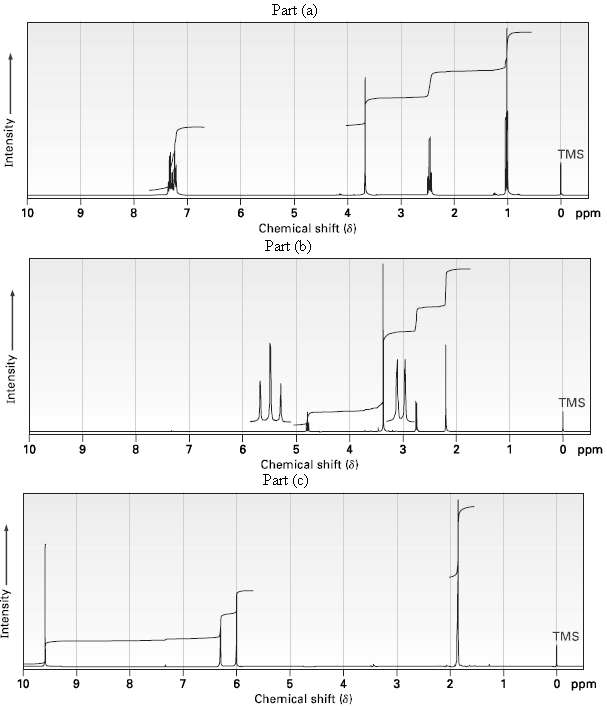
Transcribed Image Text:
Part (a) TMS 10 6. 5. Chemical shift (8) 3 0 ppm Part (b) TMS O ppm 10 9. 8. Chemical shift (8) Part (c) TMS 10 8. 4 O ppm Chemical shift (8) Intensity Intensity Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
a CHCCHCH3 b a 108 b 258 C 3...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C9H12O (b)C8H10O2 Part (a) TMS 10 O ppm Chemical shift (8) Part (b) TMS O ppm 10 8. Chemical shift (8) Intensity Intensity...
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C5H12O (b) C8H10O Part (a) TMS O ppm 10 3 2 Chemical shift (8) Part (b) TMS O ppm 10 8. 6. 4 3 2 Chemical shift (8) Inten
-
The following 1H NMR spectra are for four compounds with molecular formula C6H12O2, Identify the compounds. a. b. c. QUESTION CONTINUE TO NEXT PAGE d. 10 (ppm) frequency 10 (ppm) 10 (ppm)
-
An airplane has a mass of 5000 kg, a maximum thrust of 7000 N, and a rectangular wing with aspect ratio 6.0. It takes off at sea level with a 60 split flap as in Fig. 7.25. Assume all lift and drag...
-
How would you describe Zappos as an organization? Mechanistic? Organic? High-or low-involvement? Why?
-
A company purchases large lots of a certain kind of electronic device. A method is used that rejects a lot if 2 or more defective units are found in a random sample of 100 units. (a) What is the mean...
-
Does the interest rate on a T-bond include a default risk premium? Explain. AppendixLO1
-
Suppose that the price level in Canada is CAD16,600, the price level in France is EUR11,750, and the spot exchange rate is CAD1.35/EUR. a. What is the internal purchasing power of the Canadian...
-
Complete the following master schedule record. Click the icon to view the master schedule record. Complete the master production schedule (enter your responses as whole numbers)
-
A regional planner is studying the demographics in a region of a particular state. She has gathered the following data on nine counties. a. Is there a linear relationship between the median income...
-
Propose structures for ketones or aldehydes that have the following 1 H NMR spectra: (a) C 4 H 7 C1O ??IR: 1715 cm ?1 ? (b) C 7 H 14 O ? ? ?IR: 1710 cm ?1 ? (c) C 9 H 10 O 2 ? ? ?IR: 1695 cm ?1 ?...
-
Primary amines react with esters to yield amides: RCO2R' + R''NH2 ? RCONHR'' + R'OH. Propose a mechanism for the following reaction of an ?, ? un-saturated ester. CH .CH + CH3NH2 H3C- + CH3OH CO2CH3
-
Does a rise in national production and income per capita tend to worsen or improve air pollution, water pollution, and sanitation? Explain.
-
How could civil engineers contribute to space debris management and cleanup?
-
In what ways can the principles of resilient infrastructure be applied to design urban systems capable of withstanding natural disasters, and how do these principles contribute to the overall safety...
-
What are local variables and global variables in Python?
-
When to use a tuple vs list vs dictionary in Python? Explain some benefits of Python
-
What is Lambda Functions in Python? How do I modify a string in python?
-
Find a list of all 10 of Zappos corporate values. Pick two of the values and explain how you think those values would influence the way employees do their work.
-
Evenflow Power Co. is considering a new project that is a little riskier than the current operations of the company. Thus, management has decided to add an additional 1.5% to the company's overall...
-
Predict the shapes of the following molecules, and then predict which would have resultant dipole moments: (a) SO 2 ; (b) NH 3 ; (c) H 2 S; (d) C 2 H 4 ; (e) SF 6 ; (f) CH 2 Cl 2 .
-
Show how you might utilize the reduction of an amide, oxime, or nitrile to carry out each of the following transformations: (a) (b) (c) (d) NIH NH2 OH NH2
-
Using a different method for each part, but taking care in each case to select a good method, show how each of the following transformations might be accomplished: (a) (b) (c) (d) (e) NH2 NH2 CH3O...
-
Review the chemistry of amines given in earlier sections and provide a specific example for each of the previously illustrated reactions.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost
Computer Aided Architectural Design Futures 2001 Volume I 1st Edition - ISBN: 0792370236 - Free Book

Study smarter with the SolutionInn App


