Propose a structure for a molecule C 14 H 12 that has the following 1 H NMR
Question:
Propose a structure for a molecule C14H12 that has the following 1H NMR spectrum and has IR absorptions at 700, 740, and 890 cm?1:
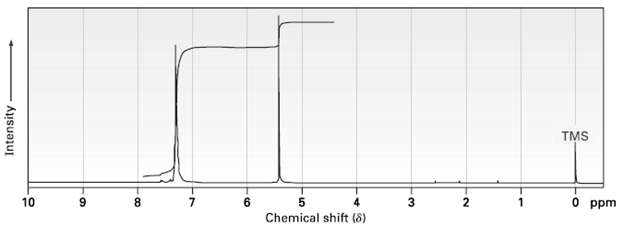
Transcribed Image Text:
TMS 3 5 Chemical shift (8) 10 O ppm 8 7 Intensity-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The compound has nine degrees of unsaturation The H NMR ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C9H12O (b)C8H10O2 Part (a) TMS 10 O ppm Chemical shift (8) Part (b) TMS O ppm 10 8. Chemical shift (8) Intensity Intensity...
-
Propose a structure for an alcohol with molecular formula C5H12 O that has the 1H NMR spectrum given in Fig. 9.46. Assign the chemical shifts and splitting patterns to specific aspects of the...
-
Propose a structure for a compound with molecular formula C 4 H 8 O that exhibits the following 13 C NMR and FTIR spectra. Carbon NMR 67.7- 25.4- 80 30 20 100 90 70 60 40 10 Chemical shift (ppm) 100...
-
Private carriage is more important in the motor carrier segment of our transportation industry than any of the other four major modal segments. What factors have contributed to private carriage...
-
How do the basic human rights - free consent, privacy, freedom of conscience, freedom of speech, and due process - apply to professional football players and the safety risks described here?
-
Sam is in his final year of college and is trying to schedule his courses for the year. He has narrowed his search to 16 courses, each of which is offered in at least one time slot (out of a possible...
-
Prepare a three- to five-minute presentation for your district manager and the other conference attendees that justifies your costs as developed.
-
Lisa Meilo works for Pacific Company, which pays its employees time- and- a- half for all hours worked in excess of 40 per week. Meilos pay rate is $ 37 per hour. Her wages are subject to federal...
-
Using the information from your balance sheet and income statement, calculate the LEVERAGE RATIO for year 2. Round answers to two decimal places.
-
Bonds 1. Municipal Bonds - Municipal bonds are haircut per Exhibit 1 based on both their time to maturity and scheduled maturity at date of issue. 2. Corporate Bonds - Corporate bonds are haircut...
-
Propose structures for aromatic compounds that have the following 1 H NMR spectra: (a) C 8 H 9 Br IR: 820 cm ?1 (b) C 9 H 12 Br IR: 750 cm ?1 (b) C 11 H 16 Br IR: 820 cm ?1 TMS 10 8. 6. 0 ppm...
-
Aromatic substitution reactions occur by addition of an electrophile such as Br+ to the aromatic ring to yield an allylic carbocation intermediate, followed by loss of fl. Show the structure of the...
-
The XYZ Corporation was bankrupt. It was decided to liquidate the firm. The following claims were presented: How much would each class of claimant get on the dollar? a. If the fixed assets were...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
Identify key factors that have contributed to the labor shortage and its effect on the hospitality industry workforce. LO1
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
Consider the reaction: A(g) 2 B(g). The graph plots the concentrations of A and B as a function of time at a constant temperature. What is the equilibrium constant for this reaction at this...
-
Oils containing highly unsaturated acids like linolenic acid undergo oxidation in air. This reaction, called oxidative rancidity, is accelerated by heat, explaining why saturated fats are preferred...
-
Give equations to show the reactions of sodium stearate with (a) Ca2+ (b) Mg2+ (c) Fe3+
-
Several commercial laundry soaps contain water-softening agents, usually sodium carbonate (Na2CO3) or sodium phosphate (Na3PO4 or Na2HPO4). Explain how these water-softening agents allow soaps to be...
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


