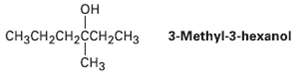
Reaction of HBr with (R)-3-methyl-3-hexanol leads to racemic 3-brorno-3-mcthylhcxane.Explain. OH CH3CH2CH2CCH2CH3 3-Methyl-3-hexanol H3
Question:
Reaction of HBr with (R)-3-methyl-3-hexanol leads to racemic 3-brorno-3-mcthylhcxane.Explain.
Transcribed Image Text:
OH CH3CH2CH2CCH2CH3 3-Methyl-3-hexanol ČH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
The chiral tertiary alcohol R3methyl3hexanol reacts with H...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
GME Bank has hired you to manage the bank's loans. Currently they have $400,000 in checking deposits and a reserve ratio of 25%. Use the bank balance sheet to answer the questions below. Assets...
-
Reaction of HBr with 2-methyipropenc yields 2-hromo-2-methylpropane, what is the structure of the carbocation formed during the reaction? Show the mechanism of the reaction. CH CHBr C=CH2 + HBr H...
-
Reaction of HBr with 3-methylcyclohexene yields a mixture of four products: cis- and trans-1-bromo-3-methylcyclohexane and cis- and trans-1-bromo- 2-methylcyclohexane. The analogous reaction of HBr...
-
According to a study conducted by the Gallup organization, the proportion of Americans who are afraid to y is 0.10. A random sample of 1100 Americans results in 121 indicating that they are afraid to...
-
Why was there a need to train first-line supervisors? What results of that training program did ConAgra observe?
-
What are the look through rules? To whom do they apply and for what purposes? What taxable items are subject to these rules?
-
Why is motivating employees a crucial part of theme park management? LO.1
-
Comment on the ethics of the following situations: a. A food warehouse club advertises savings up to 30 percent after a survey showed a range of savings from 2 to 30 percent below average prices for...
-
\ table [ [ \ table [ [ Batance Sheets ] , [ December 3 1 , 2 0 2 4 and 2 0 2 3 ] ] , 2 0 2 4 , 2 0 2 3 ] , [ { \ table [ [ Assets ] , [ Current assets: ] ] } ] , [ \ table [ [ Current assets: ] , [...
-
A 0.917-g sample of canned tuna was analyzed by the Kjeldahl method. A volume of 20.59 mL of 0.1249 M HCl was required to titrate the liberated ammonia. Calculate the percentage of nitrogen in the...
-
(S)-2-Butanol slowly racemizes on standing in dilute sulfuric acid.Explain. CH3CH2CHCH3 2-Butanol
-
Treatment of 1 .bromo-2-deuterio-2-phenylethane with strong base leads to a mixture of deuterated and non-deuterated phenylethylenes in an approximately 7: 1 ratio.Explain. Br (CH3)3CO 7:1 ratio O- ...
-
At the beginning of 2018, Silver Corporation has a $95,000 capital loss carryforward from 2017. During 2018, the corporation sells land, held for four years, and realizes an $80,000 gain. Silver has...
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
prepare financial statements, i.e. profit and loss account and balance sheet, from the trial balance.
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
What is the effect of a change in volume on a chemical reaction (that includes gaseous reactants or products) initially at equilibrium?
-
Show how Claisen condensations could be used to make the following compounds. (a) (b) (c) (d) C Ph CHCHC-CH-CH --,, C-ocH,CH,
-
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base. (a) Ethyl acetoacetate (b) Pentane-2,4-dione (c) Ethyl a-cyanoacetate (d)...
-
Show how the following compounds can be made using the malonic ester synthesis. (a) 3-phenylpropanoic acid (b) 2-methylpropanoic acid (c) 4-phenylbutanoic acid (d) Cyclopentanecarboxylic acid
-
Only need help on 4B and 5. Exercise 9-21 Breakeven Planning; Profit Planning (LO 9-2, 9-3] Connelly Inc., a manufacturer of quality electric ice cream makers, has experienced a steady growth in...
-
A project with an initial cost of $32,000 is expected to provide cash flows of $12,900, $13,100, $16,200, and $10,700 over the next four years, respectively. If the required return is 8.1 percent,...
-
A company that is expecting to receive EUR 500,000 in 60 days is considering entering into an FX futures contract to lock an exchange rate to USD for the transaction. The FX rate on the contract is...

Study smarter with the SolutionInn App


