Show the elimination products of thesereactions: Br ELOH a) CH,CH,CHCH,CH, + CH;CH20 ,, CI CI . EIOH
Question:
Show the elimination products of thesereactions:
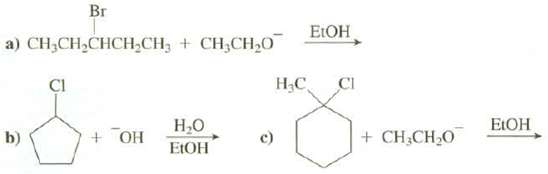
Transcribed Image Text:
Br ELOH a) CH,CH,CHCH,CH, + CH;CH20 Н,С, CI CI Н.О EIOH + OH b) + CH,CH20 EIOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
An elimination reaction occurs whe...View the full answer

Answered By

Ankur Swami
I am a lover of physics. I always thinks that how things are happened towards us. How this universe was created and how its working. So I choose physics to grow my career. So those students who are interested in science and want to learn science in a different way can come with me.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
1. Predict the elimination products of the following reactions. When two alkenes are possible, predict which one will be the major product. Explain your answers, showing the degree of substitution of...
-
Predict the elimination products of the following reactions, and label the major products. (a) cis-1-bromo-2-methylcyclohexane + NaOCH3 CH3OH (b) trans-1-bromo-2-methylcyclohexane + NaOCH3 in CH3OH
-
Show that Gaussian elimination can be performed on A without row interchanges if and only if all leading principal sub matrices of A are nonsingular. [Hint: Partition each matrix in the equation A...
-
+9.33 x 10 C +91 Find the net force on 92. 0.180 m +4.22 x 10 C +92 0.230 m- -8.42 x 10 C 93 F = force exerted on q2 by 91 F3 = force exerted on 92 by 93 F = 10.9 N F3 = 6.04 N Remember: Forces...
-
Conundrum Ltd manufactures furniture. Due to a fire in the administrative offices, the accounting records for November of the current year were partially destroyed. You have been able to piece...
-
What is meant by the term fresh start accounting?
-
Explain the special issues that may arise in a purchase, and show how to account for them. AppendixLO1
-
In the month of June, Paula's Beauty Salon gave 3,500 haircuts, shampoos, and permanents at an average price of $30. During the month, fixed costs were $16,800 and variable costs were 80% of sales....
-
Assume that your parents wanted to have $160,000 saved for college by your 18th birthday and they started saving on your first birthday. They saved the same amount each year on your birthday and...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Arrange these nucleophiles in order of decreasing rate of reaction with iodomethane and explain your answer. Problems using online three-dimensional molecular models
-
Show the products, including stereo chemistry, of these eliminationreactions. CH;CH2 Br Br Br b) H C-CH + CH,CH20 Ph ELOH Ph C-C EIO H+ OH Ph H2O Ph
-
The IFAC is attempting to harmonize auditing standards and practices. What do you think are some of the barriers to this harmonization? What are the benefits of their work being successful?
-
The following post-closing trial balance was drawn from the accounts of Spruce Timber Co. as of December 31, 2011. Transactions for 2012 1. Acquired an additional \(\$ 10,000\) cash from the issue of...
-
Bankers Trust (BT) was one of the most powerful and profitable banks in the world in the early 1990s. Under the stewardship of chairman Charles Sanford Jr., it had transformed itself from a staid...
-
Hammond Inc. experienced the following transactions for 2011, its first year of operations: 1. Issued common stock for \(\$ 80,000\) cash. CHECK FIGURES b. Net Income: \(\$ 62,520\) Total Assets:...
-
Following are the current prices and last years prices of a gallon of regular gas at a sample of 14 gas stations. Can you conclude that the median price is different now from what it was a year ago?...
-
A sample of nine men participated in a regular exercise program at a local gym. They were weighed both before and after the program. The results were as follows. Can you conclude that the median...
-
What changes, if any, will the perishability of the inventory have on the capability of a company in using a level strategy. Consider, for example, a meat market and a fast-food restaurant.
-
Find the market equilibrium point for the following demand and supply functions. Demand: 2p = - q + 56 Supply: 3p - q = 34
-
Evaluate each using the values given. 2(x - y)(y + y); use x = 6, and y = 1
-
The hormone cortisone contains C, H, and O, and shows a molecular ion at M+ = 360.1937 by high-resolution mass spectrometry. What is the molecular formula of cortisone? (The degree of unsaturation of...
-
Halogenated compounds arc particularly easy to identify by their mass spectra because both chlorine and bromine occur naturally as mixtures of two abundant isotopes. Chlorine occurs as 35C1 (75.8%)...
-
By knowing the natural abundances of minor isotopes, its possible to calculate the relative heights of M+ arid M + 1 peaks. If 13C has a natural abundance of 1.10%, what are the relative heights of...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....
-
Alado fis istirmerfs Tat likifond 205L [ridont inip lanod whadtinion? hingend is antan Qultit foer avdeed Divdasit errem yodichiders Etexlpoges Getmare nelp
-
The limitation on the deduction of business interest does not apply to non-corporate taxpayers. course hero True or False explain?

Study smarter with the SolutionInn App


