Water is in the pipe shown in Fig. 3.26. Calculate the pressure at point A in kPa(gage).
Question:
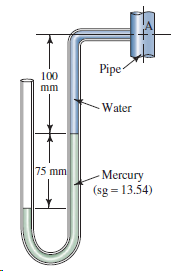
Transcribed Image Text:
Pipe 100 mm - Water 75 mm Mercury (sg = 13.54)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
p A 1354 981 kN...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the compound manometer shown in Fig. 3.31, calculate the pressure at point A. Oil (sg = 0.90) Water 125 mm 475 mm 250 mm 50 mm Mercury (sg = 13.54)
-
a. Determine the gage pressure at point A in Fig. 3.36. b. If the barometric pressure is 737 mm of mercury, express the pressure at point A in kPa(abs). Water 215 mm Mercury (sg = 13.54) 600 mm
-
If the pressure at point A in Fig. 11.29 is 300 kPa, compute the volume flow rate of water at 10°C delivered to the tank. 2.5 m Standard elbow Flow DN 40 Schedule 40 steel pipe 25 m Factory...
-
Does the customer buying process end when a customer buys some merchandise? Explain your answer.
-
Ling Ltd. and Tseng Ltd., two corporations of roughly the same size, are both involved in the manufacture of canoes and sea kayaks. Each company depreciates its plant assets using the straight-line...
-
An office supply company conducted a survey before marketing a new paper shredder designed for home use. In the survey, 80% of the people who used the shredder were satisfied with it. Because of this...
-
Household energy expenditure. Refer to the annual total household expenditure on energy from 2011 to 2020, Exercise 11.29 (p. 661). The annual total household expenditure (in millions) data on gas,...
-
The intangible assets section of Redeker Company at December 31, 2010, is presented below. Patent ($70,000 cost less $7,000 amortization)..... $63,000 Franchise ($48,000 cost less $19,200...
-
Check my work mode : This shows what is correct or incorrect for the work you have com requirea imrormauon [The following information applies to the questions displayed below.] The following...
-
A musical instruments retailer has 10,000 point-of-sale transactions out of which 1500 sales included both items of electric guitars and guitar cases, and out of which 750 had sales of new strings....
-
Determine the pressure at the bottom of the tank in Fig. 3.25. 1.2 m --- --- -- -- Air 200 kPa (gage) Oil 15m/ (sg = 0.80) 2.6 m Water 2 m
-
For the differential manometer shown in Fig. 3.27, calculate the pressure difference between points A and B. The specific gravity of the oil is 0.85. 10 in 32 in Oil Water 9 in el
-
The heat capacity at constant volume of hydrogen sulfide at low pressures is C v [kJ/ (mol??C)] = 0.0252 + 1.547 x 10 ?5? T ? 3.012 X 10 ?9 T 2? where T is in ?C. A quantity of H 2 S is kept in a...
-
Salinger Company estimates that total factory overhead costs will be $70,000 for the year. Direct labor hours are estimated to be 10,000. a. For Salinger Company, determine the predetermined factory...
-
SCS receives on average 1 data package every 1/50 seconds, with a standard deviation of 1/50 seconds, and processes them using its single powerful computing unit, which can process data packages in...
-
Suppose that we pay workers $25 per day.We value processed orders at $4 per order and the number of orders each worker can process is worker 1 - 8 orders, worker 2 - 7 orders, worker 3 - 6 orders,...
-
How do I imagine that I am the administrator of a midsize long-term care facility with an outdated information system and I have been given thetaskto planand managethe integration of a new database...
-
You are negotiating a five - year contract with a new customer. The contract could be larger than any previous contracts your company has had. Which would be your best negotiation style?
-
The concentration of a certain drug in the bloodstream t hours after being administered is approximately Use the differential to approximate the changes in concentration for the following changes in...
-
l ask this second time correnct answer is 38,01 can we look pls Consider a non-conducting rod of length 8.8 m having a uniform charge density 4.5 nC/m. Find the electric potential at P, a...
-
Draw the products of each of the following acid-base reactions, and then predict the position of equilibrium in each case: (a) (b) NaH
-
A hiker caught in a thunderstorm loses heat when her clothing becomes wet. She is packing emergency rations which if completely metabolized will release 35 kJ of heat per gram of rations consumed....
-
Predict the products obtained when 1-pentyne reacts with each of the following reagents: (a) H 2 SO 4 , H 2 O, HgSO 4 (b) 9-BBN followed by H 2 O 2 , NaOH (c) Two equivalents of HBr (d) One...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...

Study smarter with the SolutionInn App


