a. Calculate and graph the hydrogen radial wave function R 2p (r) over the interval 0
Question:
a. Calculate and graph the hydrogen radial wave function R2p(r) over the interval 0 ≤ r ≤ 8aB.
b. Determine the value of r (in terms of aB) for which R2p(r) is a maximum.
c. Example 41.3 and Figure 41.8 showed that the radial probability density for the 2p state is a maximum at r = 4aB. Explain why this differs from your answer to part b.
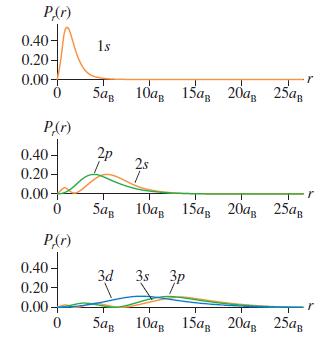
Figure 41.8
Transcribed Image Text:
P(r) 0.40- 1s 0.20- 0.00- 5а, 10а, 15ав 20а, 25а, PAr) 0.40 2p 25 0.20- 0.00+ 5ар 10а, 15ав 20а, 25ая PAr) 0.40- 0.20- 0.00+ 3d 3s 3p 5а, 10ав 15ад 20а, 25ар
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
Solve a From Equation 417 the 2p radial wave function is The graph of R 2p r is seen to have a singl...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
In Exercise 11, compare your answer to part (a) with your answer to part (b). How do outliers affect the range of a data set? Data from exercise 11: The depths (in inches) at which 10 artifacts are...
-
From your answer to the preceding problem, would it be possible for two galaxies with stars evenly distributed to pass right through each other?
-
Determine the value of C in the network shown in fig 11.50 in order for the circuit to be in resonance. 4 cos 2t V 4
-
A stream of particles of one size are 80% converted (SCMIash diffusion control, uniform gas environment) on passing through a reactor. If the reactor is made twice the size but with the same gas...
-
A vertical, frictionless piston-cylinder device contains a gas at 180 kPa absolute pressure. The atmospheric pressure outside is 100 kPa, and the piston area is 25 cm2. Determine the mass of the...
-
Assignment 1 Prepare the necessary journal entries on the books of P&G Company to record the following transactions, assuming a perpetual inventory system (you may omit explanations): P&G purchased...
-
Web-based exercise. Find an example of a poor graphic. One possible source is the CHANCE Web site at Dartmouth College. In particular, the Chance News section, www.dartmouth.edu/~chance/chance news/...
-
Allowance Method of Accounting for Bad DebtsComparison of the Two Approaches Kandel Company had the following data available for 2010 (before making any adjustments): Accounts receivable, 12/31/10...
-
[The following information applies to the questions displayed below.] OFC Company of Kansas City prints business forms and other specialty paper products, such as writing paper, envelopes, note...
-
Which of the graphs in Fig. Q25.12 best illustrates the current I in a real resistor as a function of the potential difference V across it? Explain. Figure Q25.12 (a) (b) (c) (d)
-
In general, an atom can have both orbital angular momentum and spin angular momentum. The total angular momentum is defined to be J(vector) = L(vector) + S(vector). The total angular momentum is...
-
Prove that the radial probability density peaks at r = a B for the 1s state of hydrogen.
-
Explain how an LLC or an S corporation represents the "best of both worlds" in terms of business ownership.
-
In this scenario you are the Manager of a Home and Community Care organization in Victoria. Your organization provides oversees support services for individuals in their homes. The individuals that...
-
find the wind speed and direction for the following parcels. Important note: To determine the arc tangent using the Google calculator, click the box marked "Inv" so that it is a light gray instead of...
-
As consumers we're connected to an instant feed or live updates, breaking news, and messages. We believe that when we post something on social media, we will get instant feedback from friends. What...
-
Describe the process for screening candidates for ethics. Outline which job candidate factors are illegal to consider when hiring. Explain how to obtain accurate behavior information from resumes,...
-
In relation to Operation management, sustainability and supply chain management explain the following terms; What is the overall objective of scheduling? What is JIT? What is a Lean producer? Discuss...
-
The Assembly Department of A-Okay Company uses the weighted average cost method and had 700 units in work in process that were 60% complete at the beginning of the period. During the period, 4,980...
-
On July 1, 2011, Flashlight Corporation sold equipment it had recently purchased to an unaffiliated company for $480,000. The equipment had a book value on Flashlights books of $390,000 and a...
-
A 100 g ball moving to the right at 4.0 m/s collides head-on with a 200 g ball that is moving to the left at 3.0 m/s. a. If the collision is perfectly elastic, what are the speed and direction of...
-
Old naval ships fired 10 kg cannon balls from a 200 kg cannon. It was very important to stop the recoil of the cannon, since otherwise the heavy cannon would go careening across the deck of the ship....
-
Old naval ships fired 10 kg cannon balls from a 200 kg cannon. It was very important to stop the recoil of the cannon, since otherwise the heavy cannon would go careening across the deck of the ship....
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


