Figure P 41.42 shows a few energy levels of the mercury atom. a. Make a table showing
Question:
Figure P 41.42 shows a few energy levels of the mercury atom.
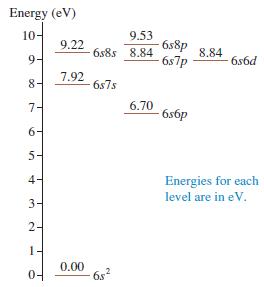
a. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate the photon wavelength, in nm.
b. What minimum speed must an electron have to excite the 492-nm-wavelength blue emission line in the Hg spectrum?
Transcribed Image Text:
Energy (eV) 10- 9.53 6s8p 6s7p 9.22 6s8s 8.84 8.84 9- 6s6d 8- 7.92 6s7s 7- 6.70 6s6p 6- 5- 4- Energies for each level are in eV. 3- 2- 1- 04 0.00 6s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Visualize Solve a We need to use the condition l 1 to ...View the full answer

Answered By

Surbhi kapoor
Finance has always been a subject of my interest. I have been indulged in helping my classmates with this subject right from the beginning of my CA cirriculum. Helping students gives me immense amount of satisfaction. Also as I have been helping my friends from a long time with this subjects of finance, accounting and cost accounting, it has developed me to make myself more expressive about the concepts of subjects. And I also feel that I understand the problem area in a particular question. This quality makes me to better understand the student's confusion and try to answer correctly.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
Figure P 41.41 shows the first few energy levels of the lithium atom. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate a. The wavelength, in...
-
The first three energy levels of the fictitious element X were shown in FIGURE P38.56. An electron with a speed of 1.4 10 6 m/s collides with an atom of element X. Shortly afterward, the atom emits...
-
The first three energy levels of the fictitious element X are shown in FIGURE P38.56. a. What is the ionization energy of element X?b. What wavelengths are observed in the absorption spectrum of...
-
From the densities of the lines in the mass spectrum of krypton gas, the following observations were made: Somewhat more than 50% of the atoms were krypton-84. The numbers of krypton-82 and...
-
The average atmospheric pressure on earth is approximated as a function of altitude by the relation Patm = 101.325 (1 - 0.02256z)5.256, where Patm is the atmospheric pressure in kPa and z is the...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning investories. The company has two manufacturing departments-Molding and Fabrication. It started, completed, and sold...
-
Counting employed people. A news article says: More people were working in America in June than in any month since the end of 1990. If thats not what you thought you read in the papers last week,...
-
Ashton Fleming has decided to document and analyze the accounts payable process at S&S so the transition to a computerized system will be easier. He also hopes to improve any weaknesses he discovers...
-
The market return, based on a broad market index (i.e. the S&P 500), is estimated to be 8%. Calculate Company XYZs required return if the risk-free rate is 1% and the stocks beta is 1.25 (round your...
-
Applebee's is the largest casual dining chain in the world, with over 1800 locations throughout the U. S. and also in 20 other countries. The menu features beef, chicken, and pork items, as well as...
-
Suppose you put five electrons into a 0.50-nm-wide one dimensional rigid box (i.e., an infinite potential well). a. Use an energy-level diagram to show the electron configuration of the ground state....
-
The ionization energy of an atom is known to be 5.5 eV. The emission spectrum of this atom contains only the four wavelengths 310.0 nm, 354.3 nm, 826.7 nm, and 1240.0 nm. Draw an energy-level diagram...
-
P627 ETHICS PROBLEM Bond rating agencies have invested significant sums of money in an effort to determine which quantitative and nonquantitative factors best predict bond defaults. Furthermore, some...
-
PART 1: DIGITAL TECHNOLOGY: Describe the key digital technology groups studied in this course and include a discussion of two examples for each group. PART 2: SOCIAL MEDIA: As studied in this course,...
-
Doing a strategic analysis of GraceKennedy Limited, What is the current level of its economic performance, an indication of the factors responsible for the current performance and recommendations for...
-
Dynamic capability is the ability for change and manage corporate learning. It allows an enterprise to adapt, develop and respond to future opportunities and discontinuous technologies. Innovation...
-
What potential solutions can organizations try to help support the adoption of a CDSS? In other words, what are some ways an organization can address the factors of implementation obstruction that...
-
Identify and briefly describe and discuss the three most important factors in building and maintaining trust among virtual global team members. Include in your discussion how you can leverage these...
-
Use the internet to find at least five marketing research firms that perform survey research. List and describe each firm briefly.
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
You are given the equation used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation. b. Draw the before-and-after pictorial...
-
You are given the equation used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation. b. Draw the before-and-after pictorial...
-
A pendulum is formed from a small ball of mass m on a string of length L. As FIGURE CP10.69 shows, a peg is height h = L/3 above the pendulums lowest point. From what minimum angle θ...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


