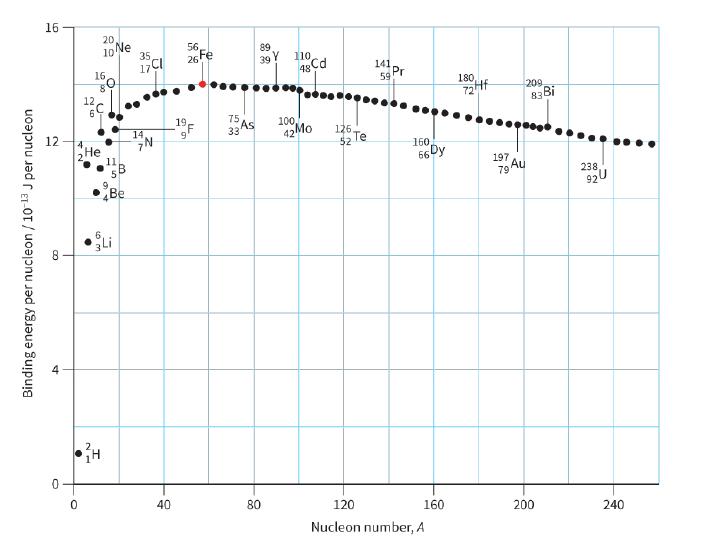
a. Explain why hydrogen 1 1 H (proton) cannot appear on the graph shown in Figure 29.4.
Question:
a. Explain why hydrogen 11 H (proton) cannot appear on the graph shown in Figure 29.4.
b. Use Figure 29.4 to estimate the binding energy of the nuclide 147 N.
Transcribed Image Text:
16 20 10 Ne 35 56, 26Fe 89 110 17CI 39 16 48 Cd 141 Pr 59 180 12 209 19, 75 As 100, 12 14 33 42Mo 126 $2 Te Не 1. 5B 160 66 Dy 197 Au 79 Be 238 920 40 80 120 160 200 240 Nucleon number, A ET Binding energy per nucleon / 10 13 J per nucleon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
a Hydrogen11 11H is a isotope of hydrogen that contains 11 nucleons 1 proton and 10 neutrons ...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
Estimate the binding energy of a KC1 molecule by calculating the electrostatic potential energy when the K+ and CI ions are at their stable separation of 0.28nm. Assume each has a charge of magnitude...
-
Estimate the binding energy of the H2 molecule by calculating the difference in kinetic energy of the electrons between when they are in separate atoms and when they are in the molecule, using the...
-
Estimate the binding energy of a KCl molecule by calculating the electrostatic potential energy when the K+ and CI- ions are at their stable separation of 0.28 nm. Assume each has a charge of...
-
What are the costs of healthcare, where does the money come from, and where is it spent?
-
When Ruby borrowed $2300, she agreed to repay the loan in two equal payments, to be made 90 days and 135 days from the day the money was borrowed. If interest is 9.25% on the loan, what is the size...
-
The market for peanut butter in Nutville is monopolistically competitive and in long-run equilibrium. One day, consumer advocate Skippy Jif discovers that all brands of peanut butter in Nutville are...
-
4.0
-
1. Use appropriate descriptive statistics to summarize the transmission failure data. 2. Develop a 95% confidence interval for the mean number of miles driven until transmission failure for the...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
In a brief essay (approximately one page or 250-300 words), provide your selected business venture and share how the use of the BMC can help your new business succeed. I wanted to pick event planner...
-
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium 3 1 H and deuterium 2 1 H. The following equation shows the process: 3 1 H + 2 1 H 4 2 He + 1 1...
-
The initial activity a sample of 1 mole of radon-220 is 8.02 10 21 s 1 . Calculate: a. The decay constant for this isotope b. The half-life of the isotope.
-
Use a CAS to explore the integrals for various values of p (include noninteger values). For what values of p does the integral converge? What is the value of the integral when it does converge? Plot...
-
4. Thinking Ahead (2 points): Project 1 involves the analysis of a bicycle pedal. Consider the bicycle shown below. If a rider places their full weight on the pedal when it is in the horizontal...
-
On January 1, 2023, Martineau Corp. issued a 5-year, 5% installment note payable for $118,000 to finance upgrading its current equipment. The company's year end is December 31. The repayment of...
-
Multiply. 2 x-x-2 3x-3 2 x+2x-3 x+1 Simplify your answer as much as possible.
-
Explain the processes of querying a relational database and define Big Data and explain its basic characteristics. Compare and contrast the major types of networks. - Identify the fundamentals of...
-
42. Explain why the inequality x - x + 1 < 0 has the empty set as the solution set.
-
The National Highway Traffic Safety Administration (NHTSA) collects traffic safety-related data for the U.S. Department of Transportation. According to NHTSA?s data, 10,426 fatal collisions in 2016...
-
Extend Algorithms 3.4 and 3.5 to include as output the first and second derivatives of the spline at the nodes.
-
Consider the entangled wave function for two photons, Assume that the polarization operator PË i has the properties PËÏ i i (H) = Ï i (H) and PË i Ï i (V ) = + Ï i...
-
Evaluate the commentator [P x + P 2 x , P 2 x ] by applying the operators to an arbitrary function f (x).
-
Revisit the double-slit experiment of Example Problem 17.2. Using the same geometry and relative uncertainty in the momentum, what electron momentum would give a position uncertainty of 2.50 10 10...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...
-
*Prepare the plant assets section of Amphonie's balance sheet at December 31, 2021 using the information below. At December 31, 2020, Amphonie Company reported the following as plant assets. Land $...

Study smarter with the SolutionInn App


