Table 29.3 gives the masses (in u) of several particles. (Avogadro constant N A = 6.02
Question:
Table 29.3 gives the masses (in u) of several particles. (Avogadro constant NA = 6.02 × 1023 mol−1.) Use the table to determine to three significant figures:
a. The mass in kg of a helium-4 nucleus
b. The mass in gram (g) of 1.0 mole of uranium-235 nuclei.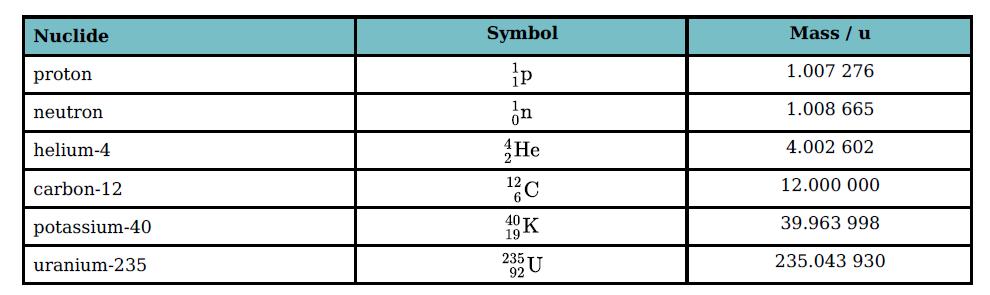
Transcribed Image Text:
Nuclide Symbol Mass / u proton 1.007 276 neutron 1.008 665 helium-4 He 4.002 602 carbon-12 12.000 000 potassium-40 40K 39.963 998 19 uranium-235 235 235.043 930
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a The mass of a helium4 nucleus can be calculated by adding the masses of its two protons and two ne...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
Pass the Journal entries, for the following transactions on the dissolution of the firm of P and Q after various assets (other than cash) and outside liabilities have been transferred to Realisation...
-
In Figure, three particles of mass m = 23 g are fastened to three rods of length d = 12 cm and negligible mass. The rigid assembly rotates around point O at angular speed w = 0.85 rad/s. About O,...
-
A rigid, mass less rod has three particles with equal masses attached to it as shown in Figure P11.45. The rod is free to rotate in a vertical plane about a frictionless axle perpendicular to the rod...
-
Identifying the leadership strategies that were employed by the organization described in the case study, how they were employed, and any additional strategies that could be employed to reflect...
-
Krista borrowed $14 000. The loan is to be repaid by three equal payments due in 120, 240, and 260 days from now. Determine the size of the equal payments at 7% with a focal date of today.
-
Many schemes for price discriminating involve some cost. For example, discount coupons take up the time and resources of both the buyer and the seller. This question considers the implications of...
-
R3
-
Compute Ke and Kn under the following circumstances: a. D1 = $5.00, P0 = $70, g = 8%, F = $7.00. b. D1 = $0.22, P0 = $28, g = 7%, F = $2.50. c. E1 (earnings at the end of period one) = $7, payout...
-
QS 14-6 (Static) Computing ending work in process inventory LO P1 Compute ending work in process inventory for a manufacturer using the following information. Raw materials purchased Direct materials...
-
1. According to Porter's framework, what generic strategy was Airborne Express pursuing? Was this a sound strategy in the context of the air express industry? 2. What were the strengths of Airborne...
-
The equation shows the radioactive decay of radon-222. 222 86 Rn 218 84 Po + 4 2 + r Calculate the total energy output from this decay and state what forms of energy are produced. (Mass of 222 86...
-
A carbon-12 atom consists of six protons, six neutrons and six electrons. The unified atomic mass unit (u) is defined as 1/12 the mass of the carbon-12 atom. Calculate: a. The mass defect in...
-
Recognizing the challenges business faces in managing business-government relations in different countries.
-
You want to retire after working 35 years with savings in excess of $1,100,000. You expect to save $3,300 a year for 35 years and earn an annual rate of Interest of 11%. (Round your answer to 2...
-
FOLLOW ALL INSTRUCTIONS AND GENERATE YOUR CODE AFTER READING THE JUNIT TESTS, THAT IS ALL THE METHODS AND CONSTRUCTORS YOU USE SHOULD BE BASED ON THE JUNIT TESTS PROVIDED. I HAVE ATTATCHED THE JAVA...
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
According to a 2018 article in Esquire magazine, approximately 70% of males over age 70 will develop cancerous cells in their prostate. Prostate cancer is second only to skin cancer as the most...
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
How would the results of Stern and Gerlach be different if they had used a Mg beam instead of an Ag beam?
-
How would the results of Stern and Gerlach be different if they had used a homogeneous magnetic field instead of an inhomogeneous field?
-
Discuss whether the results shown in Figure 17.7 are consistent with local realism. Figure 17.7 300 250 200 150 100 50 -8 -6 -4 -2 4 6. 8. Detector 2 position/mm Coincidence counts
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


