The table shows the received count rate when a sample of the isotope vanadium-52 decays. a. i.
Question:
The table shows the received count rate when a sample of the isotope vanadium-52 decays.
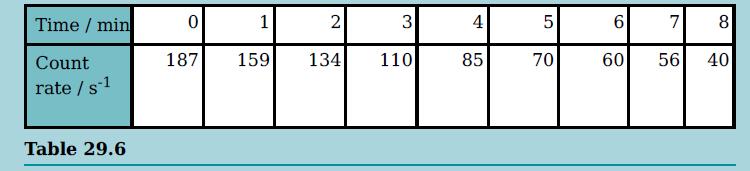
a. i. Sketch a graph of the count rate against the time.
ii. Comment on the scatter of the points.
b. From the graph, determine the half-life of the isotope.
c. Describe the changes to the graph that you would expect if you were given a larger sample of the isotope.
Transcribed Image Text:
Time / min 1 2 3 4 6. 7 8 Count 187 159 134 110 85 70 60 56 40 rate / s1 Table 29.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a i Sketch a graph of the count rate against the time Timemin 0 1 2 3 4 5 6 7 8 Count rate s 1 187 1...View the full answer

Answered By

Kamalakanta Nayak
I have completed B.Tech and MBA. I have been teaching students both offline and online mode. I teach both physics and math for CBSE, ISC, IB, AP-1,2,C, IGCSE, GCSE, AQA, EDEXCEL, CIE, OCR. I prepare students for NEET, JEE, and MCAT. I work at any student's level from any Curriculum with concept driven Real-life examples and Goal-Oriented Teaching, making the concepts so simple that you can understand it regardless of prior knowledge. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam. Get guidance and the opportunity to learn from experienced...
1. I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject. Contact me in solutioninn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advice related to tutoring and how it works here.thank you.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
A sample of an isotope for which = 0.10 s 1 contains 5.0 10 9 undecayed nuclei at the start of an experiment. Determine: a. The number of undecayed nuclei after 50 s b. Its activity after 50 s.
-
When a sample of customer accounts receivable is selected for vouching debits, auditors will vouch them to a. Sales invoices with shipping documents and customer sales invoices. b. Records of...
-
When a sample of 10 cereal boxes of a well-known brand was reweighed, it gave the following weights (in ounces): a. What is the sample mean? And the sample variance? b. If the true mean weight per...
-
Explain how community service staff members can monitor the impact of work undertaken and/or services provided in line with the scope of their own work role?
-
Compute the present value of a debt of $708.13 eighty days before it is due if money is worth 5.3%.
-
Two athletes of equal ability are competing for a prize of $10,000. Each is deciding whether to take a dangerous performance-enhancing drug. If one athlete takes the drug, and the other does not, the...
-
1.0
-
A company prepares financial statements in order to summarize financial information. Below are a list of financial statements and a list of descriptions. Financial Statements a. Balance sheet b....
-
Joirnalozs a purchase of sale of the common stock on August 1 and Motor Inc buys about 1000 shares of asthma, stock for $35,000 cash on December 1 and Motor Inc sell stock investments for $38,000 in...
-
Use the following information to answer questions #1-2. Computers for your store were purchased each month from January through April. However, the cost varied because of the manufacturer's price...
-
Use the information given in the fusion section, to determine the binding energy (in MeV) per nucleon of each particle in the following fusion reaction: 2 1 H + 1 1 p 3 2 He
-
A sample of carbon-15 initially contains 500,000 undecayed nuclei. The decay constant for this isotope of carbon is 0.30 s 1 . Calculate the initial activity of the sample.
-
Prairie Computers Limited (PCL) has three projects it could implement with the following cost and NPV profiles. However, PCL has only $1 million available to spend on capital projects. Define capital...
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
Suppose that the mean retail price per gallon of regular grade gasoline in the United States is $3.43 with a standard deviation of $.10 and that the retail price per gallon has a bell-shaped...
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
Does the bond length of a real molecule depend on its energy? Answer this question by referring to Figure 18.7. The bond length is the midpoint of the horizontal line connecting the two parts of V...
-
Why can the angular momentum vector lie on the z axis for two-dimensional rotation in the xy plane but not for rotation in three-dimensional space?
-
How does the total energy of the quantum harmonic oscillator depend on its maximum extension?
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


