a. Draw a diagram of an electrochemical cell consisting of a Cr 3+ /Cr half-cell and a
Question:
a. Draw a diagram of an electrochemical cell consisting of a Cr3+/Cr half-cell and a Cl2/Cl– half-cell.
b. Use the data in Appendix 2 to calculate the cell voltage.
c. Which half-cell is the positive pole?
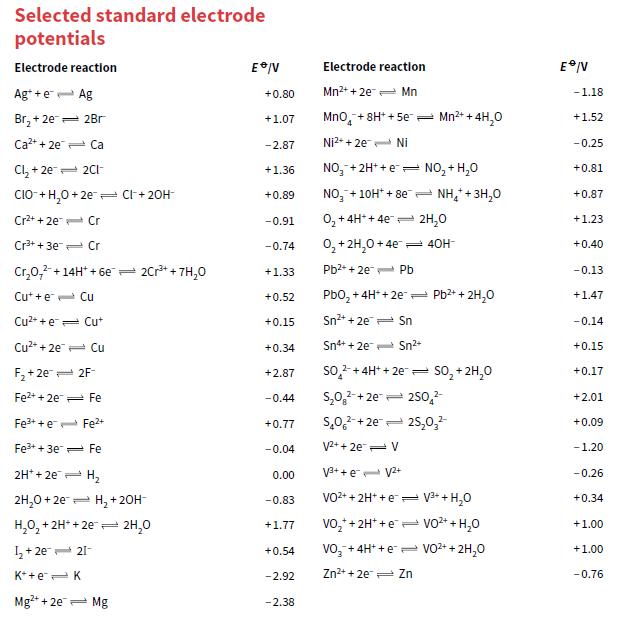
Transcribed Image Text:
Selected standard electrode potentials Electrode reaction Electrode reactlon Ag* +e- Ag Mn2+ + 2e- Mn +0.80 -1.18 Br, + 2e= 2Br +1.07 Mno, + 8H* + 5e- Mn2+ + 4H,0 +1.52 Ca2+ + 2e Ca -2.87 Ni2+ + 2e Ni - 0.25 Cl, + 2e 2CI- NO,-+ 2H+ +e - NO, + H,0 +0.81 +1.36 CIO- + H,0 + 2e Cl+ 20H NO, + 10H* + 8e- NH,+3H,0 +0.89 +0.87 Cr2+ + 2e Cr -0.91 0, + 4H* + 4e= 2H,0 +1.23 Cr3+ + 3e Cr 0, + 2H,0 + 4e 40H- -0.74 +0.40 Cr,0,2-+14H* + 6e- 2Cr+ + 7H,0 Pb2* + 2e Pb +1.33 -0.13 Cut + e- Cu Pbo, +4H* + 2e= Pb2+ + 2H,o +0.52 +1.47 Cu* + e= Cut +0.15 Sn2+ + 2e Sn -0.14 Cu2+ + 2e Cu +0.34 Sn+ + 2e Sn2+ +0.15 F, + 2e 2F so, + 4H* + 2e so, + 2H,0 +2.87 +0.17 Fe2+ + 2e Fe -0.44 S,0,-+ 2e= 250,2 +2.01 Fe3+ + e Fe2+ s,0, +2e= 25,0, +0.77 +0.09 Fe+ + 3e= Fe -0.04 V2+ + 2e- V -1.20 2H* + 2e H, V3+ + e- V2+ 0.00 -0.26 2H,0 + 2e= H, +20H- VO2+ + 2H* +e = V3+ + H,0 +0.34 -0.83 H,0, + 2H* + 2e 2H,0 vo,* + 2H* +e vo2* + H,0 +1.00 +1.77 I, + 2e 21- Vo,-+ 4H* + e e VO2+ + 2H,0 +0.54 +1.00 K* +e=K -2.92 Zn2+ + 2e Zn -0.76 Mg+ + 2e- Mg -2.38
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a The diagram of an electrochemical cell consisting of a Cr3Cr halfcell and a Cl2Cl halfcell ca...View the full answer

Answered By

Mary Boke
I have teached the student upto class 12th as well as my fellow mates.I have a good command in engineering,maths and science.I scored 90+ marks in 10th and 12th in maths.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
a. Draw a diagram of an electrochemical cell consisting of a Mn 2+ /Mn half-cell and a Pb 2+ /Pb half-cell. b. Use the data in Appendix 2 to calculate the cell voltage. c. Which half-cell is the...
-
Use the data in Appendix 3 to calculate the equilibrium constant for the reaction Agl(s) Ag+(aq) + I2(aq) at 25C. Compare your result with the Ksp value in Table 16.2.
-
The data in Appendix D (available at www.uvm.edu/~dhowell/fundamentals8/DataFiles/Add.dat) are actual data on high school students. What is the 75th percentile for GPA in these data? (This is the...
-
On 28 April 2020, Mr Guna, CEO of Econ Engineering Malaysia, proposed to complete an abandoned boiler project that no one had dared to revive. He knew that the project was 60% complete before it was...
-
Let z be a random variable with a standard normal distribution. Find the indicated probability and shade the corresponding area under the standard normal curve. P( 0.45 z 2.73)
-
The production and home delivery of a pizza is a relatively straightforward and simple process. Develop a fishbone diagram to identify potential defects and opportunities for poor quality in this...
-
Why do project teams create time-phased budgets? What are their principal strengths?
-
Joanjim Corporation was organized on January 1, 2012. It is authorized to issue 20,000 shares of 6%, $40 par value preferred stock, and 500,000 shares of no-par common stock with a stated value of $2...
-
[The following information applies to the questions displayed below) At year-end December 31, Chan Company estimates its bad debts as 0.90% of its annual credit sales of $859.000. Chan records its...
-
Ramada Company produces one golf cart model. A partially complete table of company costs follows: Required: 1. Complete the table. 2. Ramada sells its carts for $1,200 each. Prepare a contribution...
-
Show, with the aid of a diagram, how you would measure the E Q value for the half-cell shown by the equation: VO 2+ + 2H + + e V 3+ + H 2 O
-
State the direction of the electron flow in the electrochemical cells represented by the following pairs of half-equations. Use the data in Appendix 2 to help you. a. F 2 + 2e 2F and Mn 2+ + 2e ...
-
List the major sources of prospects.
-
Following the example shown in (a) below, indicate the effects of the listed transactions on the assets, liabilities, and stockholders equity of John Dallmus, certified public accountant, a...
-
What effect does the ordering of a search tree have on the efficiency of the search? What effect does it have on the quality of the results? How would order affect the way that depth-first search or...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Sales Revenue Equipment Common Stock Notes Payable Retained Earnings...
-
Smart Sports is also planning to launch a range of drinks products. The products have been developed by Hydration Labs Ltd and are designed to be sold as powders that dissolve easily in water. They...
-
Baucom Company accepted credit cards in payment for \(\$ 6,850\) of services performed during March 2011. The credit card company charged Baucom a 4 percent service fee. The credit card company paid...
-
Define the CECL model for accounts receivable. On what does it base the estimate of the allowance for uncollectible accounts?
-
Write a function that reads a Float24_t value: Float24_t float24_read(void) A legitimate float24 value string is of the form: "mantissabexponent" where the mantissa (m) and the exponent (e) may have...
-
In an adiabatic compression of one mol of an ideal gas with C V ,m = 5/2 R, the temperature rises from 278 K to 450. K. Calculate q, w, H, and U.
-
Can the following compound be prepared via a Williamson ether synthesis? Explain your answer.
-
Calculate H o R and U o R for the oxidation of benzene (g). Also calculate -U R.
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


