Look at Figure 29.27. a. What is the retention time of the compound shown? b. What is
Question:
Look at Figure 29.27.
a. What is the retention time of the compound shown?
b. What is the approximate relative molecular mass of the compound shown?
c. How would the compound be identified?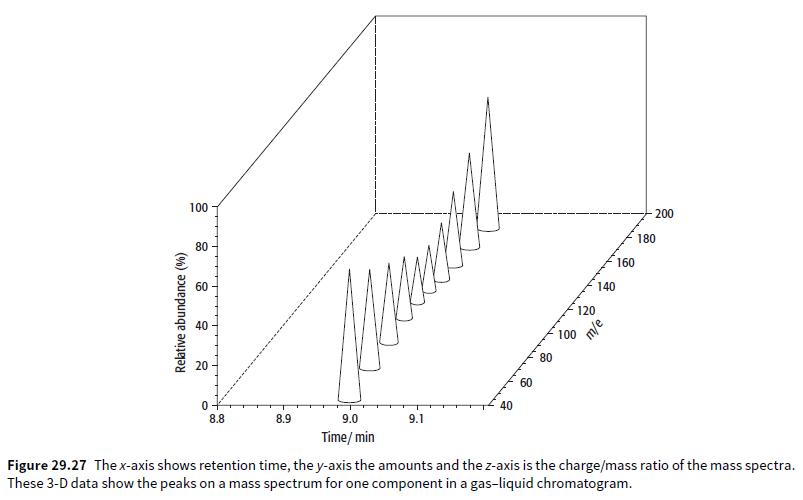
Transcribed Image Text:
100 200 80 180 160 60 140 120 40 100 20 80 60 40 8.8 8.9 9.0 9.1 Time/ min Figure 29.27 Thex-axis shows retention time, the y-axis the amounts and the z-axis is the charge/mass ratio of the mass spectra. These 3-D data show the peaks on a mass spectrum for one component in a gas-liquid chromatogram. Relative abundance (%)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a We have to answer what is the retention time of the compound shown The retention time ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Use this information to compute the following: FunTime Cruiseline offers nightly dinner cruises departing from several cities on the eastern coast of the United States including Charleston,...
-
A large distributor of oil-well drilling equipment operated over the past two years with EOQ policies based on an annual holding cost rate of 28%. Under the EOQ policy, a particular product has been...
-
(a) Make a graph showing the retention time of each peak in Figure 24-25 in chromatograms B, F, and C as a function of position along the line BC. Predict the retention times for solvent compositions...
-
Swish Designs specialises in designing commercial office space in Auckland. The CEO, Ralph Polo has reviewed the financial results and has noticed that operating profits were below budget. He also...
-
The owner of a sporting goods store is considering remodeling the store to carry a larger inventory. The cost of remodeling and additional inventory is $60 000. The expected increase in net profit is...
-
Discuss efforts by companies to circumvent governmental regulations. Is the use of legal loopholes ethical?
-
What is manufacturing lead time? Name and describe each of its elements. LO.1
-
A brilliant young scientist is killed in a plane crash. It is anticipated that he could have earned $240,000 a year for the next 50 years. The attorney for the plaintiffs estate argues that the lost...
-
The Shirt Shop had the following transactions for T-shirts for Year 1, its first year of operations: Jan. 20 Purchased 510 units @ $ 9 = $ 4,590 Apr. 21 Purchased 310 units @ $ 11 = 3,410 July 25...
-
The database Applicants.xlsx contains a description of an MBA applicant pool. a. How many records are in this database? b. How many fields are in this database? c. W hat decision was made on the top...
-
Look at Figure 29.28. a. Calculate the relative molecular mass of leucine enkephalin (C 28 H 37 N 5 O 7 ) using relative atomic masses. (A r values C = 12.0, H = 1.0, N = 14.0, O = 16.0) b. i. How is...
-
a. List the ions responsible for the M, [M + 2] and [M + 4] peaks in a mass spectrum of dibromomethane. b. What would be the mass-to-charge ratio and relative abundances of the major peaks with the...
-
Lets continue to look at the DJIA discussed in problem 26. Table 2.6.8 shows 23 daily observations of the value of the DJIA, with 22 observations of the net change from one observation to the next,...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
Velky s.r.o. (a Czech company) invests 1,200,000 schillings in a foreign subsidiary on January 1, Year 1. The subsidiary commences operations on that date and generates net income of 400,000...
-
When you weigh yourself on good old terra firma (solid ground), your weight is 142 lb. In an elevator your apparent weight is 121 lb. What are the direction and magnitude of the elevator's...
-
For each of the following pairs of compounds, identify the higher boiling compound and justify your choice: a. b.
-
Refer to Figure 1.10 and explain why (U/V) T is generally small for a real gas. Figure 1.10 Ideal gas Real gas Tranlaition rv-0 (4)A
-
Can a gas be liquefied through an isenthalpic expansion if J T = 0?
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Golden Corporation's current year income statement, comparative balance sheets, and...
-
Glencove Company makes one model of radar gun used by law enforcement officers. All direct materials are added at the beginning of the manufacturing process. Information for the month of September...
-
Larren Buffett is concerned after receiving her weekly paycheck. She believes that her deductions for Social Security, Medicare, and Federal Income Tax withholding (FIT) may be incorrect. Larren is...

Study smarter with the SolutionInn App


