Look at the mass spectrum of germanium, Ge. a. Write the isotopic formula for the heaviest isotope
Question:
Look at the mass spectrum of germanium, Ge.
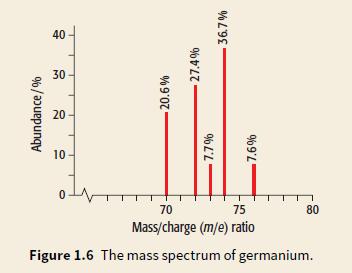
a. Write the isotopic formula for the heaviest isotope of germanium.
b. Use the % abundance of each isotope to calculate the relative atomic mass of germanium.
Transcribed Image Text:
40 30 20 10 70 75 80 Mass/charge (m/e) ratio Figure 1.6 The mass spectrum of germanium. Abundance/% 20.6% 27.4% 96 L'L 36.7%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a According to the mass spectrum of germanium Ge provided in the question the isotopic formul...View the full answer

Answered By

SHAKIL AHMED
I worked as a homework/assignment helper for more than 4 years. I also worked as a teaching assistant for Bachelor's students. I loved teaching them. Interaction with the students help me to grow as a teacher and I learned a lot while teaching them.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
The mass spectrum of methyl isobutyl ether does not show a peak due to inductive cleavage, in contrast to the mass spectrum of di-sec-butyl ether (Eq. 12.31). Use what you know about carbocation...
-
The mass spectrum of 1-butanol shows an intense daughter ion peak at m/z 5 31. Explain how this peak might arise.
-
The mass spectrum of n-octane shows a prominent molecular ion peak (m z 114). There is also a large peak at m/z 57, but it is not the base peak. The mass spectrum of 3,4-dimethylhexane shows a...
-
Timco is considering the construction of a new retail outlet. The construction cost will be 400000. Net working capital will increase by 10000. The depreciation is 10 year MACRS. The new location...
-
Give atomic symbols for each element. a. Sodium b. Argon c. Nitrogen d. Radon
-
The following transactions relate to Gordon's business for the month of July 2011: 1. Bought goods on credit from Watson. 2. Sold some goods for cash. 3. Sold some goods on credit to Moon. 4. Sent a...
-
Milden Company is a merchandiser that plans to sell 12,000 units during the next quarter at a selling price of $100 per unit. The company also gathered the following cost estimates for the next...
-
Suppose that on January 1 Brothers Golf Company paid cash of $35,000 for equipment that is expected to remain useful for five years. At the end of five years, the equipments value is expected to be...
-
Question 2: Shannon Mosher died in Minnesota. Her close relatives were her mother, her ex-husband, and their two children, Drake Mosher and Jeribeth Peters. Shannon left the following estate: Item...
-
Selected transactions from the journal of June Feldman, investment broker, are presented below. Instructions a. Post the transactions to T-accounts. b. Prepare a trial balance at August 31, 2020....
-
Solid sodium carbonate reacts with aqueous hydrochloric acid to form aqueous sodium chloride, carbon dioxide and water. Na 2 CO 3 + 2HCl 2NaCl + CO 2 + H 2 O a. Rewrite this equation to include...
-
This question is about two transition metals, hafnium (Hf) and zirconium (Zr). a. Hafnium forms a peroxide whose formula can be written as HfO 3 .2H 2 O. Use the A r values below to calculate the...
-
Calculate this line integral by Stokess theorem for the given F and C. Assume the Cartesian coordinates to be right-handed and the z-component of the surface normal to be nonnegative. F = [e y , 0, e...
-
The following accounts appear in the ledger of Sheridan Ltd. after the books are closed at December 31 ( in thousands). Share Capital-Ordinary, no par, 1 stated value, 400,000 shares authorized;...
-
1. Let f(x) 223-9x2. (a) Find all critical points for f(x).
-
Sadie's ski shop sells ski and boots. Skis are sold for $300 per pair and have associated Variable Costs of $150 per pair. Boots are sold for $200 per pair with an associated Variable Expense of $65...
-
How do you apply what is learned in the Science of Branding 1 5 - 1 , Key Insights Regarding Global Brand Strategies Based on Research Findings to improve on the brand?
-
CASH MANAGEMENT Dr. Umburgh noticed that the $100.00 check made to Trenton Medical Supplies has not cleared for four months. What type of check is the outstanding check referred to as? a. Stale-dated...
-
Sachs Brandss defined benefit pension plan specifies annual retirement benefits equal to 1.6% service years final years salary, payable at the end of each year. Angela Davenport was hired by Sachs...
-
Smiths Family Fashions implemented a balanced scorecard performance measurement system several years ago. Smiths is a locally owned clothing retailer with fashions for men, women, teens, and...
-
Identify the reagents you would use to achieve each of the following transformations: a) Convert tert-butyl bromide into a primary alkyl halide b) Convert 2-bromopropane into 1-bromopropane
-
Identify the reagents necessary to accomplish each of the transformations shown below. If you are having trouble, the reagents for these transformations appear on page 444, but you should first try...
-
Identify the reagents you would use to accomplish each of the following transformations: (a) Convert 2-methyl-2-butene into a monosubstituted alkene (b) Convert 2,3-dimethyl-1-hexene into a...
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


