Look at the two infra-red spectra below: a. Which one of the infra-red spectra is that of
Question:
Look at the two infra-red spectra below:
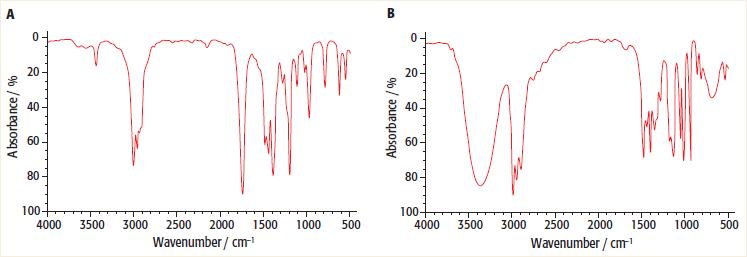
a. Which one of the infra-red spectra is that of butanone and which one is of butan-2-ol?
b. Explain your reasoning in part a.
Transcribed Image Text:
В 20 20- 40 40- 60 60 80 08 100 4000 100 3500 3000 2500 2000 1500 1000 500 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber / cm- Wavenumber / cm A bsorbance/ %
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a Image A is for Butanone image B is for Butan 2ol b In image A there ...View the full answer

Answered By

Manasa D K
I am a MSc graduate, cleared lectureship eligibility test. I has a 5 years of experience in teaching chemistry for 11th and 12th, BSc and MSc graduates. My skills include, proficiency in a wide range of teaching methods and techniques. Extensive hands on experience in the practice of chemistry and solid grasp of its theories. Comprehensive knowledge and understanding of the subject to creat, execute and analyse student assessments.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Look at the two histograms, created from 2009 real estate data taken from the Ventura County Star, and decide whether you think the standard deviation of home prices in Agoura, California (A), was...
-
One of the basic financial principles is that the value of any asset (whether it be a stock, a bond, or a firm as a whole) is the present value of that asset's future cash flows. As you learned in...
-
Which one of the following press types is usually associated with the highest production rates in sheet-metal-stamping operations: (a) adjustable bed, (b) open-back inclinable, (c) press brake, (d)...
-
Find the laurents series f(z)= 1/(z-1)(z-2) (i) |z| <1 (ii) 1 2
-
The following is based on information from The Wolf in the Southwest: The Making of an Endangered Species by David E. Brown (University of Arizona Press). Before 1918, the proportion of female wolves...
-
If you leave 100 in the bank earning 6% interest, at the end of n years you will have 100 1.06n. Draw a graph of this amount over the next 20 years.
-
When traveling, one always has a choice of airlines. However, which should you choose? Many attributes are worthy of consideration, some of which are cost, airline miles, number of hops (intermediate...
-
On December 31, 2012, Grando Company sells production equipment to Fargo Inc. for $50,000. Grando includes a 1-year warranty service with the sale of all its equipment. The customer receives and pays...
-
The following information pertains to Julia & Company: March 1 Beginning inventory = 34 units @ $5.40 March 3 Purchased 21 units @ 3.80 March 9 Sold 29 units @ 8.60 What is the cost of goods sold for...
-
The Chartered Financial Analyst (CFA) designation is the de facto professional certification for the financial industry. Employers encourage their prospective employees to complete the CFA exam....
-
An alcohol has the molecular formula C 3 H 8 O. When warmed with an alkaline solution of iodine it forms a yellow precipitate. a. Name the yellow precipitate. b. Draw the displayed formula of the...
-
Use data from the table below of characteristic infra-red absorptions in organic molecules to answer the following question. One of the three spectra labelled A to c below is produced when ethanal is...
-
The substitution or addition methods can also be used to solve a system of three linear equations in three variables. Consider the following system. x + y + z = 7 x - y + 2z = 9 -x + 2y + z = 4 The...
-
Write a brief statement that interprets the confidence interval. Choose the correct answer below. A. There is a 99% chance that the true value of the population mean weight of newborn girls will fall...
-
Transcribed image text: If estimated annual factory overhead is $1,072,500; overhead is applied using direct labor hours, estimated annual direct labor hours are 275,000 actum March factory overhead...
-
Your firm has limited capital to invest and is therefore interested in comparing projects based on the profitability index (PI), as well as other measures. What is the PI of the project with the...
-
The following rates are applicable to annual payroll in British Columbia Question 17 options: 1234 1.95% x total B.C. remuneration 1234 2.925% x (B.C. remuneration - $500,000) 1234 Tax Rate 1234...
-
Assume that different groups of couples use a particular method of gender selection and each couple gives birth to one baby. This method is designed to increase the likelihood that each baby will be...
-
In 2021, internal auditors discovered that PKE Displays, Inc. had debited an expense account for the $350,000 cost of equipment purchased on January 1, 2018. The equipments life was expected to be...
-
Find the equations of the ellipses satisfying the given conditions. The center of each is at the origin. Passes through (2, 2) and (1, 4)
-
A 1.50 mole sample of an ideal gas at 28.5C expands isothermally from an initial volume of 22.5 dm 3 to a final volume of 75.5 dm 3 . Calculate w for this process a. For expansion against a constant...
-
Derive the equation (H/T) V = C V + V/ from basic equations and definitions.
-
Consider the reaction TiO 2 (s) + 2 C(graphite) + 2 Cl 2 (g) 2 CO(g) + TiCl 4 (l) for which ÎH o R ,298 K = 80. kJ mol 1 . Given the following data at 25°C, Assume that the heat capacities...
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


