Use data from the table below of characteristic infra-red absorptions in organic molecules to answer the following
Question:
Use data from the table below of characteristic infra-red absorptions in organic molecules to answer the following question.
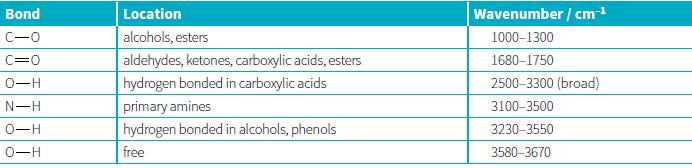
One of the three spectra labelled A to c below is produced when ethanal is analysed in an infra-red spectrophotometer:
Which infra-red spectrum is most likely to be produced by ethanal? Give three reasons for your choice.
Transcribed Image Text:
Location alcohols, esters Bond Wavenumber / cm-1 C-0 1000-1300 C=0 aldehydes, ketones, carboxylic acids, esters 1680-1750 0-H hydrogen bonded in carboxylic acids 2500-3300 (broad) primary amines hydrogen bonded in alcohols, phenols N-H 3100-3500 0-H 3230-3550 0-H free 3580-3670
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
Acetaldehyde another name for ethanol is a basic organic molecule having the chemical formula CH3CHO ...View the full answer

Answered By

Agrima Nigsm
In my capacity as an lecturer in biology at a school, I play a crucial role in educating students and shaping their brains to become the future generation of biologists. I am in charge of conducting research, teaching, and providing services to the school community. I have to teach as part of my job. I create and instruct classes on a variety of subjects, such as physiology, genetics, ecology, and animal behavior. I make an effort to make sure that your pupils have a solid grasp of the material and the abilities needed to utilize this knowledge in their future employment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Use information from Table 16.2 to answer the following questions about sound in air. At 20oC the bulk modulus for air is 1.42 X 105 Pa and its density is 1.20 kg/m3. At this temperature, what are...
-
Use the following payoff matrix to answer the following questions. Suppose this is a one-shot game:a. Determine the dominant strategy for each player. If such strategies do not exist, explain why...
-
Which information below is most likely to be incorporated by reference in Form 10-K Select one: A. Controls and procedures. B. Audited financial statements and supplementary financial information. C....
-
On 1 January 2022, ABC Company issued 10,000 shares of common stock for $100,000. On 31 December 2022, the company declared and paid dividends of $10,000. Calculate the earnings per share and the...
-
What is your favorite color? A large survey of countries, including the United States, China, Russia, France, Turkey, Kenya, and others, indicated that most people prefer the color blue. In fact,...
-
Auditors want to select a sample of 300 invoices from the 9,000 available. How could they do this?
-
Consider the data from Problem 14-10. The human resources department requires that non-dimensional scaling be applied to make your decision. Rate the individual attributes using Equations (14-1) and...
-
COMPUTE CASH PROVIDED BY OPERATING ACTIVITIES Kennington Company's condensed income statement for the year ended December 31, 20-2, was as follows: Net sales........$800,000 Cost of goods...
-
Denumap/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fims stclaircollege.ca%252Fwebapps%25 Youtube Maps ter 12 Lab Ethe Saved Help Save & Exit Submit Problem 12-5A Dividend...
-
1. Explain Jamess behavior in terms of the frustration model. 2. Cite a specific example of role conflict in this case. 3. What type of conflict resolution strategy is the union steward suggesting?...
-
Look at the two infra-red spectra below: a. Which one of the infra-red spectra is that of butanone and which one is of butan-2-ol? b. Explain your reasoning in part a. 20 20- 40 40- 60 60 80 08 100...
-
Copper(II) nitrate decomposes on heating. The reaction is endothermic. 2Cu(NO 3 ) 2 (s) 2CuO(s) + 4NO 2 (g) + O 2 (g) a. Draw an enthalpy level diagram (reaction profile diagram) for this reaction....
-
During your annual audit of Walker Distributing Co., your assistant, Jane Williams, reports to you that, although a number of entries were made during the year in the general ledger account Notes...
-
A storeroom is used to organize items stored in it on N shelves. Shelves are numbered from 0 to N-1. The K-th shelf is dedicated to items of only one type, denoted by a positive integer A[K]....
-
CASES CASE 10.1 Money in Motion Jake Nguyen runs a nervous hand through his once finely combed hair. He loosens his once perfectly knotted silk tie. And he rubs his sweaty hands across his once...
-
(3.8) Axiom, Definition of false false = true (3.9) Axiom, Distributivity of over : (pq) p=q
-
The board of directors of Unilever has been impressed by the presentation you did, and they further instructed you to conduct a more insightful investigation about the Sri Lankan market. They have...
-
The sample space listing the eight simple events that are possible when a couple has three children is {bbb, bbg, bgb, ogg, gbb, gbg, ggb, ggg}. After identifying the sample space for a couple having...
-
For financial reporting, Clinton Poultry Farms has used the declining-balance method of depreciation for conveyor equipment acquired at the beginning of 2018 for $2,560,000. Its useful life was...
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
Which of the following systems are open? a) A dog, b) An incandescent light bulb c) A tomato plant d) a can of tomatoes. Explain your answers.
-
Calculate S, S surroundings , and S universe per day for the air conditioned house described in Problem 5.4. Assume that the interior temperature is 65F and the exterior temperature is 99F.
-
The following heat capacity data have been reported for L-alanine: By a graphical treatment, obtain the molar entropy of L-alanine at T = 300.K.You can perform the integration numerically using...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


