The diagram shows an electrochemical cell involving two metal/metal-ion systems. The standard electrode potentials for the half-cells
Question:
The diagram shows an electrochemical cell involving two metal/metal-ion systems.
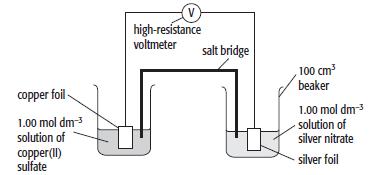
The standard electrode potentials for the half-cells are:
Ag+ + e– ⇌ Ag Eθ= +0.80 V
Cu2+ + 2e– ⇌ Cu Eθ= +0.34 V
a. Calculate a value for the cell voltage. Show your working.
b. Write the balanced ionic equation for the overall cell reaction.
c. In this reaction:
i. Which substance is oxidised? Explain your answer.
ii. Which substance is reduced? Explain your answer.
iii. In which direction do the electrons flow? Explain your answer.
d. The contents of the Cu2+/Cu half-cell are diluted with water. The contents of the Ag+/Ag half-cell are kept the same. Suggest what effect this will have on the value of the cell voltage, E. Explain your answer.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





