The table below shows the 1st ionisation energies, H i1 , in kJ mol 1, of the
Question:
The table below shows the 1st ionisation energies, ΔHi1, in kJ mol–1, of the elements in Period 3 of the Periodic Table.
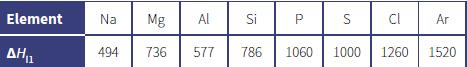
a. Explain why there is a general increase in the value of ΔHi1 across the period.
b. Explain why aluminium has a lower value of ΔHi1 than magnesium.
c. Write the electronic configuration for argon (Z = 18) using 1s2 notation.
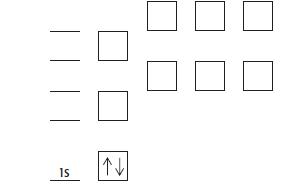
d. Copy and complete the diagram below for the 15 electrons in phosphorus by
i. Adding labels for the other subshells.
ii. Showing how the electrons are arranged..
e. Predict a value for the 1st ionisation energy for potassium, which has one more proton than argon.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





