The table lists the boiling points of some Group 15 hydrides. a. Explain the trend in the
Question:
The table lists the boiling points of some Group 15 hydrides.
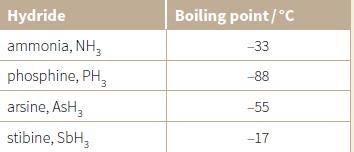
a. Explain the trend in the boiling points from phosphine to stibine.
b. Explain why the boiling point of ammonia does not follow this trend.
Transcribed Image Text:
Hydride Boiling point/°C ammonia, NH, -33 phosphine, PH, -88 arsine, AsH, -55 stibine, SbH, -17
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Solution agenerally melting and boiling points of compounds increases down the groupfrom phophine t...View the full answer

Answered By

Monish Ramesh
Tuttored mostly elementary school students privately after school and during the summer. We met in their homes or at the public library. I charged an hourly fee, and I provided any necessary materials.
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as needed.
While my students and I certainly utilized technology and the internet often during our sessions, I never tutored online or for any tutoring company, so am not familiar with the curriculums or methods used in those settings.
Tutoring one on one was very enjoyable and rewarding. My students and I had fun, and grew quite fond of one another. The extra income was a bonus. I had to retire from tutoring due to a physically handicapping disease, and miss my students very much.
I teach Chemistry since 2 years, the best experience that I would like to share is that irrespective of basics that an individual has, need to help the student in understanding the core concepts in clarity. In that way, I am able to push my students in understanding the basic logical structure of subject and excel slowly. I prefer and pump the concepts slowly rather than going in a super sonic speed. For any student (irrespective of class) acquiring interest in the subject matters rather than learning like a machine.
Read
understand
understood
register
recollect
apply
innovate
invent
these are the fundamental steps to progress a learning curve in the subject.
For the last one+ year, I have been teaching “How to teach online”
Why? Because many good tutors out there do not know of tech tools that can make online teaching more efficient and engaging.
Other than than that, I have done some sessions on “Problem Solving and Decision Making” for folks in the IT industry. Basically, I enjoy sharing experiences
The back and forth and the light-bulb moments when students see something they hadn't before are a great stimulation.
If you want an ever-varying career, then teaching is an option. BUT not knowing where you are I would say have a talk with teachers in your country.
Tutoring is something else. Usually one on one, but I prefer two students at a time, so they can spark one another off and teach one another.
BUT don't think that doing it for free or for very little is doing them a favour
I’ve had the fortunate opportunity to give some guest lectures on software build infrastructure. Also I’ve given some training on design patterns and various short sessions on various topics.
I quite enjoyed doing those actually, so far that I did consider it as a career more than once.
If iam interested in learning a new language, I will teach someone in my mother tongue and learn theirs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
The table shows the atomic number and boiling points of some noble gases. a. Use ideas about forces between atoms to explain this trend in boiling points. b. Xenon forms a number of covalently bonded...
-
Explain why a. H2O has a higher boiling point than CH3OH(65oC). b. H2O has a higher boiling point than NH3(- 33oC). c. H2O has a higher boiling point than HF (20C).
-
This table indicates the freezing and boiling points of several molecules: Molecule Freezing Point (C) Boiling Point (C) Water 0 100 Carbon tetrachloride (CCl4) - 23 77 Methane (CH4) - 182 - 164...
-
Find all values of 0, if 0 is in the interval [0, 360) and has the given function value. cot 0= -1 0= (Type your answer in degrees. Use a comma to separate answers as needed.)
-
Use your calculator to evaluate these expressions. Express the final answer in proper scientific notation. a. 456 (7.4 108) =? b. (3.02 105) ( (9.04 1015) =? c. 0.0044 0.000833 = ?
-
Cindy and Robert (Rob) Castillo founded the Castillo Products Company in 2008. The company manufactures components for personal decision assistant products and for other handheld electronic products....
-
2. On January 1, 2016, the Ben Company formed a foreign subsidiary. On February 15, 2016, Bens subsidiary purchased 100,000 local currency units (LCU) of inventory; 25,000 LCU of the original...
-
On December 31, 2007 the Robey Company accumulated the following information for 2007 in regard to its defined benefit pension plan: Service cost ................$105,000 Interest cost on projected...
-
Compute GST liability of a Company for the month of March 2021 on the following Government Services - Particulars Amount) Fee for processing of application for advance authorisation 12.000 Merchant...
-
Adam and Heidi Larrsson were delighted when Adam landed a new job with a promotion and an increased salary, but disappointed to learn that he would not be eligible for group benefits for the first 90...
-
Describe the changes that occur in the closeness and motion of the particles when: a. A solid changes to a liquid b. A liquid changes to a gas.
-
Bromine, Br 2 , and iodine monochloride, ICl, have the same number of electrons. But the boiling point of iodine monochloride is nearly 40 C higher than the boiling point of bromine. Explain this...
-
7,500 lb/h of a 50 wt% aqueous solution of FeC13 at 100C is cooled to 20C. At 100oC, the solubility of the FeC13 is 540 g/100 g of water. At 2PC, the solubility is 91.8 g/100 g water and crystals of...
-
What is an incident in which a famous person wore or used a product (not as part of a paid endorsement or ad) and it caused a buying frenzy. Explain how the manufacturer or service provider reacted
-
What is a "heavyweight project team" and how does it differ from the traditional approach used for organizing development projects at Eli Lilly?This consists of two issues:First, an evaluation of the...
-
Consider the closed-loop system shown in Figure P11.6, where the transfer function of the process is that of a second-order system, i.e. k Ts +25TS +1 G,(s)= Y sp(s) E(s) U(s) Y(s) Ge(s) Gp(s) Figure...
-
1. Do you feel we have come along way with inventory in 10 years? 2. How did COVID affect the supply chain in your current hospital? Were any of the inventory systems/topics used, or relevant or...
-
Identify at least one way in which your writing skills have improved this semester and reflect on how you might use this skill in your career. You can include research, presentation, and report...
-
On January 1, 2021, Harlon Consulting entered into a three-year lease for new office space agreeing to lease payments of $7,000 in 2021, $6,000 in 2022, and $5,000 in 2023. Payments are due on...
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
Draw all constitutional isomers with molecular formula C 5 H 10 that possess one p bond.
-
Rank the following anions in terms of increasing basicity:
-
Identify the alkene that would yield the following products via ozonolysis: a. b. c. d.
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


