Rank the following anions in terms of increasing basicity:
Question:
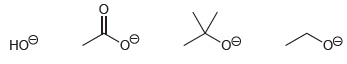
Transcribed Image Text:
ное
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Increa...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rank the following anions in order of decreasing basicity: CH O CH,o CH
-
Draw all constitutional isomers with molecular formula C 2 H 6 S, and rank them in terms of increasing acidity.
-
For each of the following compounds, rank the highlighted bonds in terms of increasing wave number: a. b. TH. 0= H.
-
Mr. Paulo Rodriguez, a fund manager of The Blackstone Group Inc. holds a portfolio with a current value of RM7.83 million. The cash index currently stands at 1,075 points. He fears that the market...
-
Show that there are inputs that force every percolateDown in heapsort to go all the way to a leaf.
-
Sketch the graph of a function that does not have a point of inflection at (c, (c)) even though "(c) = 0.
-
A company uses a tracking signal trigger of 4 to decide whether a forecast should be reviewed. Given the following history, determine in which period the forecast should be reviewed. MAD for the item...
-
Sydney Garner is considering building a 300- seat amphitheater in a popular park. After studying the market, Sydney has drawn the following conclusions: There will be one show every night during...
-
Dobbs Company issues 5%, two-year bonds, on December 31, 2017, with a par value of $200,000 and semiannual interest payments. Seniannual Period-End (0) 12/31/2017 (1) 6/30/2018 (2) 12/31/2018 (3)...
-
Your company was hired as a subcontractor for a client's "Destiny" project and awarded two main work packages within that project. Your company refers to this project internally using the cost code...
-
Which of the following is not one of the four major economic flows linking the U.S. economy with that of other nations? a. Trade flows. b. Resource flows. c. Financial flows. d. Foreign aid flows.
-
Compare a hypothetical DVC with a hypothetical IAC. In the DVC, average per capita income is $500 per year. In the IAC, average per capita income is $40,000 per year. If both countries have a savings...
-
Use the given figure to solve for the angle measurements. (6x+1) (9x+4) (6x-14)
-
Everyone at some point has had issues with time management and procrastination in their work life, academic life and social life. How have you been handling time management issues in your life? Have...
-
You want to make three peanut butter and jelly sandwiches. What is the best way to make them that's consistent with an agile mindset? Create a sandwich assembly line, applying all the peanut butter...
-
1 pts Joan Reed exchanges commercial real estate that she owns for other commercial real estate, plus $50,000 cash. The following additional information pertains to this transaction: Property given...
-
It is believed that 86% of Padres fans would have liked Trevor Hoffman to remain in San Diego to finish out his career as a San Diego Padre. You would like to simulate asking 10 Padres fans their...
-
The videos below cover why American higher education, including public colleges and universities, is so expensive. They also explore factors that have resulted in the current student loan debt...
-
Use the function (x) = 2x + 7 to find each of the following. The y-intercept of its graph
-
What is EBIT/eps analysis? What information does it provide managers?
-
Tell the number of hydrogens bonded to each carbon atom in the following substances and give the molecular formula ofeach: OH H (a) (b) CO2CH3 Ephedrine Cocaine
-
Identify the most electronegative element in each of the following molecules: (a) CH2FC1 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


