Identify the alkene that would yield the following products via ozonolysis: a. b. c. d.
Question:
a.
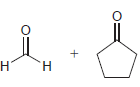
b.
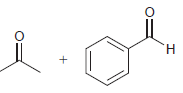
c.
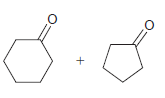
d.
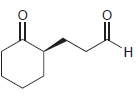
Transcribed Image Text:
Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
a...View the full answer

Answered By

Charles Okinda
students should give all the instructions concerning the challenge that they face. they will get an immediate response because I am always online.
4.90+
754+ Reviews
1483+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give an example reaction that would yield the following products. Name the organic reactant and product in each reaction. a. Alkane b. Monohalogenated alkane c. Dihalogenated alkane d. Tetra ha...
-
Identify the alkene that would give each of the following products upon ozonolysis followed by treatment with hydrogen peroxide: a. b. c. d. e. f. CH3CH2CH2COH CH3CCH CH3CCH2CH2CH2CH2CCH2CH3 OH HO +...
-
Write the structures of the alkenes that would yield the following carbonyl compounds When treated with ozone and then with dimethyl sulfide. (a) (b) (2 Mol is produced from 1 mol of alkene) (c) o...
-
Thor Bhd. (Thor) is a listed company in Malaysia, specializes in selling batteries. At 31 December 2021, Thor holds four distinct types of batteries in its warehouse. The accountant of Thor provided...
-
Show the result of performing three deleteMin operations in the heap of the previous exercise.
-
What are two of the differences between large- and small-company payroll practices?
-
What are new global challengers? What advantages do they typically possess? L01
-
Your analysis of Moen Corporations fixed asset accounts at the end of 2016 reveals the following information: 1. Moen owns two tracts of land. The first, which cost $18,000, is being held as a future...
-
Blue Company purchased a machine on July 1, 2018, for $30,800. Blue paid $220 in title fees and county property tax of $138 on the machine. In addition, Blue paid $550 shipping charges for delivery,...
-
The Primo Insurance Company is introducing two new product lines: special risk insurance and mortgages. The expected profit is $5 per unit on special risk insurance and $2 per unit on mortgages....
-
The intermediate-term external benefits of Head Start a. Focus on reducing the likelihood of the children ending up in prison. b. Focus on reducing the likelihood that the children will become...
-
Social Securitys revenue emanates from taxes on a. All income. b. Payrolls. c. Capital. d. Estates.
-
Cost of units completed and ending inventory ; FIFO method Clearwater Candy Co. had a cost per equivalent pound for the month of $4.56 for materials, $1.75 for labor, and $1.00 for overhead. During...
-
St. Cecilia's Health System's current culture may be defined by a blend of historical ideals and problems. Given its history of primarily caring for women and children, it is likely to place a...
-
For this assignment, imagine we are the supervisor of case managers who come to us for support and guidance. You have noticed a theme of questions that are frequently asked, which mostly surround...
-
Audio Partners needs to invest in the next level of technology in order to be competitive. The company is exploring the purchase of a new piece of equipment that will cost $1,500,000, at an expected...
-
Choose a real company of their choosing and will focus on ways to help increase the company's digital consumer engagements. For example, how can the company better drive increased revenue, sales,...
-
Four morally and ethically relevant principles have been examined regarding scarcity and include: Treating people with consistency through the use of a lottery or first-come first-served basis...
-
Factor each trinomial. m 2 - 11m + 60
-
Interview managers at three companies in your area about their use of ERP. How have their experiences been similar? What accounts for the similarities and differences?
-
Which of the following molecules has a dipole moment? Indicate the expected direction ofeach. (b) (d) (c) (a)
-
(a) The H C1 bond length is 136 pm. What would the dipole moment of HC1 be if the molecule were 100% ionic, H+ C1-? (b) The actual; dipole moment HC1 is 1.08 D. What is the percent ionic character...
-
Phosgene, C12C = O, has a smaller dipole moment then formaldehyde, H2C = O, even though it contains electronegative chlorine atoms in place of hydrogen. Explain
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...

Study smarter with the SolutionInn App


