Write rate equations for each of the following reactions: a. Cyclopropane propene where rate is proportional
Question:
Write rate equations for each of the following reactions:
a. Cyclopropane → propene where rate is proportional to the concentration of cyclopropane
b. 2HI(g) → H2(g) + I2(g) where rate is proportional to the square of the hydrogen iodide concentration
c.
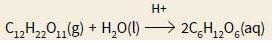
where rate is proportional to the concentration of C12H22O11 and to the concentration of H+ ions
d. 2HgCl2(aq) + K2C2O4(aq) → Hg2Cl2(s) + 2KCl(aq) + 2CO2(g), where rate is proportional to the concentration of HgCl2 and to the square of the concentration of K2C2O4.
e.
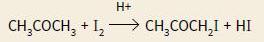
where rate is proportional to the concentration of CH3COCH3 and to the concentration of H+ ions, but the concentration of I2 has no effect on the rate.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





