Nitrogen is to be isothermally compressed at 0C from 1 bar to 100 bar. Compute the work
Question:
Nitrogen is to be isothermally compressed at 0°C from 1 bar to 100 bar. Compute the work required for this compression; the change in internal energy, enthalpy; Helmholtz and Gibbs energies of the gas; and the heat that must be removed to keep the gas at constant temperature if
a. The gas is an ideal gas.
b. The gas obeys the virial equation of state
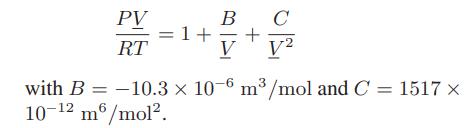
c. The gas is described by the van der Waals equation
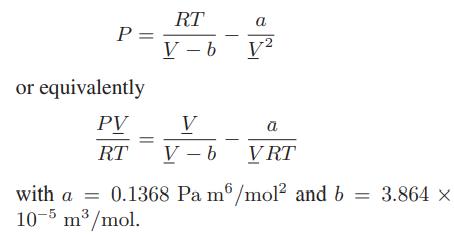
d. The gas is described by the Peng-Robinson equation of state.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





