Repeat the calculations of Problem 6.13 if the mechanical efficiency of the adiabatic turbine is only 85
Question:
Repeat the calculations of Problem 6.13 if the mechanical efficiency of the adiabatic turbine is only 85 percent.
Problem 6.13
Eighteen kilograms of the refrigerant HFC-134a at 150°C is contained in a 0.03-m3 tank. Compare the prediction you can make for the pressure in the tank with that obtained using Fig. 3.3-4. For data, see Table 6.6-1.
Fig. 3.3-4.
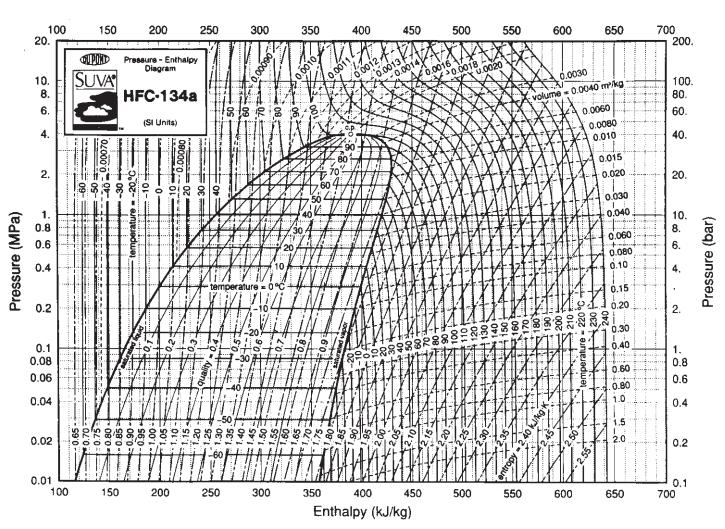
Table 6.6-1.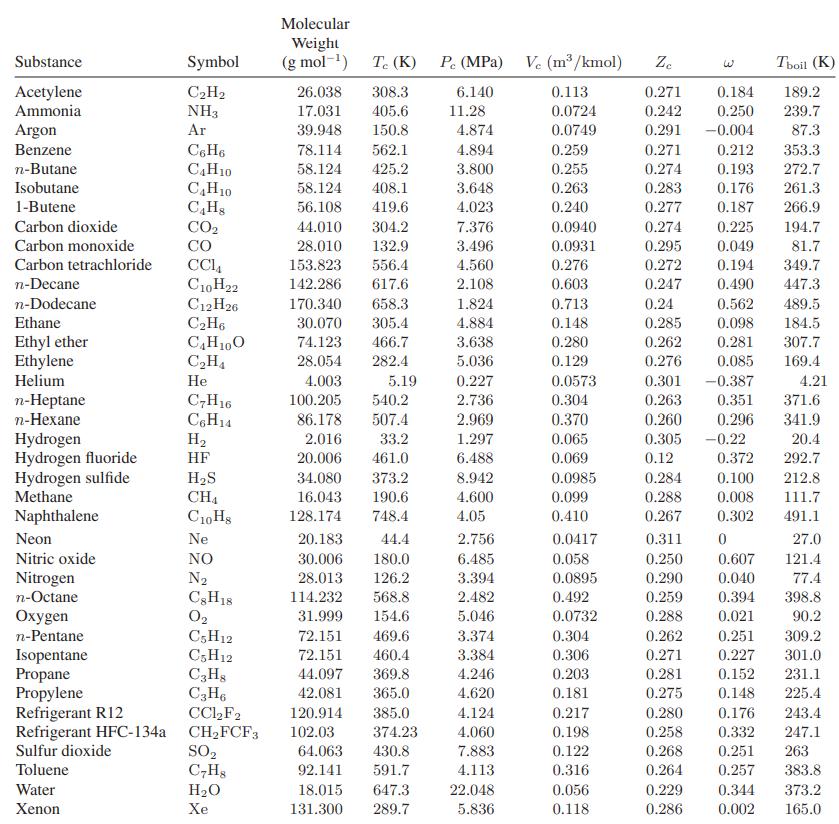
Transcribed Image Text:
Pressure (MPa) 100 20. 10. 8. 6. 4. N 2. 1. 0.8 0.6 0.4 0.2 0.1 0.08 0.06 0.04 0.02 0.01 100 150 QUPORD Pressure - Enthalpy Diagram SUVA HFC-134a (SI Units) 0.65 0.70 0.75 0.00070 -30 temperature -20°C -0.80- -0.85- 0.90- 150 200 960- 250 1.00- 1.05- 1.10- 1.15- 1.20: 200 1885 300 1.35. 1.30 21.25. 250 0.0009 temperature 0°C 1764 20 300 350 0.001 350 400 0.001 0.0013 (0.0012, -0.0014 450 400 450 8888888 Enthalpy (kJ/kg) 0.0016 0.0018 80 500 சம் 100 1104 120+ 500 0.0020 550 FOEK HEREN 140 160 335 41503 NUKITS $65 0/1 EN RECE 550 1801 600 0.0030 volume-0.0040 m²/kg 061 200 temperature 220° BEHI SE 650 0.0060 - 50 600 0.0080 0.010 0.015 0.020 0.030 0.040 0.060 ++ 0.080 0.10 0.15 0.20 0.30 0.40 HIH 0.60 0.80 1.0 O... 1.5 2.0 650 700 200. 100. 80. 60. 40. 8 20. 10. 8. 6. ✔ 2. 1. 0.8 CO CO 0.6 0.4 0.2 0.1 700 Pressure (bar)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Consider a heat engine and heat pump connected as shown in figure P.7.38. Assume TH1 = TH2 > Tamb and determine for each of the three cases if the setup satisfy the first law and/or violates the 2nd...
-
Redo Problem 6.32 using Aspen Plus Problem 6.32 Repeat the calculations of Problem 6.13 if the mechanical efficiency of the adiabatic turbine is only 85 percent. Problem 6.13 Eighteen kilograms of...
-
Redo Problem 6.13 using Aspen Plus Problem 6.13 Eighteen kilograms of the refrigerant HFC-134a at 150C is contained in a 0.03-m 3 tank. Compare the prediction you can make for the pressure in the...
-
The comparative balance sheets for Karidis Ceramics, Inc., for December 31, 209 and 208 are presented on the next page. During 209, the company had net income of $96,000 and building and equipment...
-
During a period of a few years, intense price competition characterized both the retail and the wholesale oil markets. At times, prices in the wholesale market fell below the manufacturers cost. One...
-
What is the effect of operating leverage on the volatility of a firms EBIT in response to changing sales?
-
how can the intrinsic capabilities of an operations resources influence operations strategy?
-
Anticipated sales for Sale Ride Tire Company were 36,000 passenger car tires and 16,000 truck tires. Beginning and ending finished goods inventories for both products were negligible, and thus were...
-
The following trial balance of Indigo Traveler Corporation does not balance. Indigo Traveler Corporation Trial Balance April 30, 2020 Debit Credit Cash $6,272 Accounts Receivable 5,420 Supplies 3,147...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Nitrogen is to be isothermally compressed at 0C from 1 bar to 100 bar. Compute the work required for this compression; the change in internal energy, enthalpy; Helmholtz and Gibbs energies of the...
-
The speed of propagation of a small pressure pulse or sound wave in a fluid, v S , can be shown to be equal to where is the molar density. a. Show that an alternative expression for the sonic...
-
The average cost per unit at production level x is defined as C avg (x) = C(x)/x, where C(x) is the cost of producing x units. Average cost is a measure of the efficiency of the production process....
-
Predicting Gender A study addressed the issue of whether pregnant women can correctly predict the gender of their baby. Among 104 pregnant women, 57 correctly predicted the gender of their baby...
-
Chamberson Medical Center is comparing their cash flow statements for 2022 to 2021. On the following cash flow form, what would be the cash and cash equivalents for the beginning of the year for...
-
What concept is important for effective planning and can be seen in various fields, including business and politics?
-
Dr. Powers operates a single-provider family medical practice. One medical assistant handles appointments, basic bookkeeping functions, and assists with medical records. Two additional medical...
-
Quiz 6 Fall 2019 - MGCR-211-001/002/003 edugen.wileyplus.com WileyPLUS Financial Accounting, Seventh Canadian Edition by Kimmel, Weygandt, Kieso, Trenholm, Irvine, and Burnley Help | System...
-
Which group in the carboxylate salt form of alanine is more basic, the --NH2 group or the -- CO2- group?
-
Discrete sample spaces: suppose there are N cable cars in San Francisco, numbered sequentially from 1 to N. You see a cable car at random; it is numbered 203. You wish to estimate N. (See Goodman,...
-
Chemists and engineers must be able to predict the changes in chemical concentration in a reaction. A model used for many single-reactant processes is Rate of change of concentration = -kC n where C...
-
Chemists and engineers must be able to predict the changes in chemical concentration in a reaction. A model used for many singlereactant processes is Rate of change of concentration = -kC n where C...
-
The following list gives the measured gas mileage in miles per gallon for 22 cars of the same model. Plot the absolute frequency histogram and the relative frequency histogram. 23 25 26 25 27 25 24...
-
How much money should be deposited at age 50 in order to withdraw $30000 at the end of each year for 5 years if the first withdrawal is made at age 65. The account earns 8.25% compounded quarterly....
-
Suppose you are the money manager of a $4.98 million investment fund. The fund consists of four stocks with the following investments and betas: Stock Investment Beta A $ 240,000 1.50 B 700,000 (0.50...
-
Newton Company is privately owned by four individuals. The company sells athletic shoes, clothing, and accessories. An existing piece of equipment that keeps breaking down must be replaced....

Study smarter with the SolutionInn App


