Question: Redo Problem 4.38 using Aspen Plus. Problem 4.38 Atmospheric air is to be compressed and heated as shown in the figure before being fed into
Redo Problem 4.38 using Aspen Plus.
Problem 4.38
Atmospheric air is to be compressed and heated as shown in the figure before being fed into a chemical reactor. The ambient air is at 20◦C and 1 bar, and is first compressed to 5 bar and then heated at constant pressure to 400◦C. Assuming air can be treated as a single-component ideal gas with C∗V = 21 J/(mol K), and that the compressor operates adiabatically and reversibly,
a. Determine the amount of work that must be supplied to the compressor and the temperature of the gas leaving the compressor.
b. Determine the heat load on the heat exchanger.
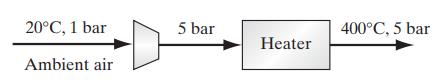
20C, 1 bar Ambient air 5 bar Heater 400C, 5 bar
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
1 Import the necessary components Aspen Plus V121 PengRobinson equation of state 2 Create the simula... View full answer

Get step-by-step solutions from verified subject matter experts


