Show that the criterion for chemical equilibrium developed in the text, for a closed system at constant
Question:
Show that the criterion for chemical equilibrium developed in the text,
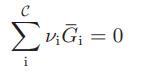
for a closed system at constant temperature and pressure, is also the equilibrium condition to be satisfied for closed systems subject to the following constraints:
a. Constant temperature and volume
b. Constant internal energy and volume
Transcribed Image Text:
C ΣvG = 0 i
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The image youve provided shows the mathematical condition for chemical equilibrium where i represents the stoichiometric coefficient for each substance and i represents the partial molar Gibbs free en...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A student is using a model to show how charge is induced. A balloon that was rubbed on animal fur and charged negatively is brought near two aluminum cans resting on two separate cups. Draw (-) signs...
-
In Sec. 8.7 we established that the condition for equilibrium between two phases is for closed systems either at constant temperature and pressure or at constant internal energy and volume. Show that...
-
a. Show that the condition for equilibrium in a closed system at constant entropy and volume is that the internal energy U achieve a minimum value subject to the constraints. b. Show that the...
-
Establish procedures to guarantee substantiation of claims for allowances. Think about how you would set up a process to ensure employee claims and allowances could be claimed. Write a step by step...
-
On January 2, Burt asked Logan to loan him money against my diamond ring. Logan agreed to do so. To guard against intervening liens, Logan received permission to file a financing statement, and Burt...
-
On 1 January 20x1, P Co entered into an agreement with Tuscany Co to acquire the net assets and business of Tuscany Co. The following transactions arose on 1 July 20x1 to execute the agreement with...
-
What effect can the conceptual and perceptual processes have on organizational behavior?
-
As a creator of advertising, what kind of return on investment did OgilvyOne get out of this promotional contest? These days, there are some extremely creative ads fighting for our attention....
-
please help Inventories Raw materials Work in process Finished goods Beginning of period $ 49,000 9,700 51,000 End of Period $ 35,000 20,400 34,000 Cost incurred for the period Raw materials...
-
Banner Ltd's budget for the four months from January to April includes the following data. 1. 2. One third of sales revenue is received one month after sale and the remainder is received two months...
-
Show that the partial molar volumes computed from Eqs. 8.6-4a and b and the partial molar enthalpies computed from Eqs. 8.6-9a and b must satisfy the Gibbs-Duhem equation. Amix V-1 a(Amix V) 1 T,P...
-
Prove that since total mass is conserved during a chemical reaction, where m i is equal to the molecular weight of species i. Also show, by direct substitution, that the first of these equations is...
-
In an effort to avoid foreclosure proceedings on struggling mortgage customers, Bank of America proposed an allowance that a jobless customer makes no payment on their mortgage for up to 9 months. If...
-
A boat leaves port and follows a course of N77E at 9 knots for 3 hr and 20 min. Then, the boat changes to a new course of S26E at 12 knots for 5 hr. Part 1 of 3 (a) How far is the boat from port?...
-
The aggregate supply curve of an economy is depicted by AS, shown in the graph on the right. Suppose that labour unions grant concessions, enabling firms to pay lower wages to their workers. Use the...
-
what is Medibank pestle analysis in term of these 2 statements? Current problem at hand deviates towards the fact that customers do not have high awareness of the health and wellbeing programs that...
-
Your company has a Microsoft 365 E5 subscription. You need to review the Advanced Analysis tab on emails detected by Microsoft Defender for Office 365. What type of threat policy should you...
-
(a) The Bright company is evaluating a project which will cost Rs 1,00,000 and will have no salvage value at the end of its 5-year life. The project will save costs of Rs. 40,000 a year. The company...
-
a. If the luminous intensity at 0 angular displacement is 3.0 mcd for the device of Fig. 1.53, at what angle will it be 0.75 mcd? b. At what angle does the loss of luminous intensity drop below the...
-
The sales department of P. Gillen Manufacturing Company has forecast sales in March to be 20,000 units. Additional information follows: Finished goods inventory, March 1 . . . . . . . . . . . . . . ....
-
Ball bearings support a shaft at points A and B (Figure P4.30). The shaft is used to transmit power between two V-belts that apply forces of 1 kN and 1.4 kN to the shaft. Determine the magnitudes and...
-
A portable music player is sitting in a docking station (Figure P4.31). The docking station has a mass of 500 g and the player, 100 g. Determine the reaction forces at the two supports. Figure P4.31...
-
Two pots of food are being cooked on a solar cooker (Figure P4.32). The smaller pot weighs 4 lb, and the larger pot weighs 9 lb. Also, due to the thermal expansion of the parabolic reflector, a...
-
Question 1. Are mortgages in the US similar to options from the perspective of the homeowner? 1. No, because defaulting does not eliminate liability. 2. No in recourse states, yes in non-recourse...
-
A risk-free 3-year annual coupon bond has a 5% coupon rate, a face value of $1,000, and trades for $950. The 1-year spot rate is 5%. The 2-year spot rate is 5.25%. The 3-year spot rate is 5.5%. What...
-
The primary purpose of the cash budget is: Select one: a. To allow the firm to anticipate the need for outside funding b. To determine the collection pattern c. To determine monthly cash receipts d....

Study smarter with the SolutionInn App


