The following vapor-liquid equilibrium data are available for the system carbon dioxide (1) + isobutane (2) at
Question:
The following vapor-liquid equilibrium data are available for the system carbon dioxide (1) + isobutane (2) at 273.15 K.
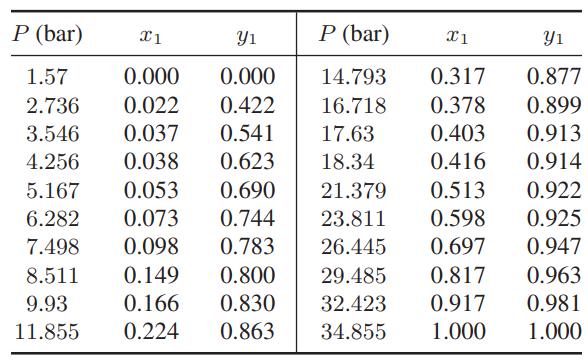
a. Find the value of the binary interaction parameter in the Peng-Robinson equation of state with the van der Waals one-fluid mixing rules that best fits these data, and plot the correlated results and experimental data on the same graph.
b. Find the partition coefficients Ki = yi/xi from both correlation and the experimental data for each species as a function of pressure, and plot all the partition coefficients as a function of pressure on a single graph.
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:




