Question: Redo Problem 10.3-12 using Aspen Plus. Problem 10.3-12 The following vapor-liquid equilibrium data are available for the system carbon dioxide (1) + isobutane (2) at
Redo Problem 10.3-12 using Aspen Plus.
Problem 10.3-12
The following vapor-liquid equilibrium data are available for the system carbon dioxide (1) + isobutane (2) at 273.15 K.
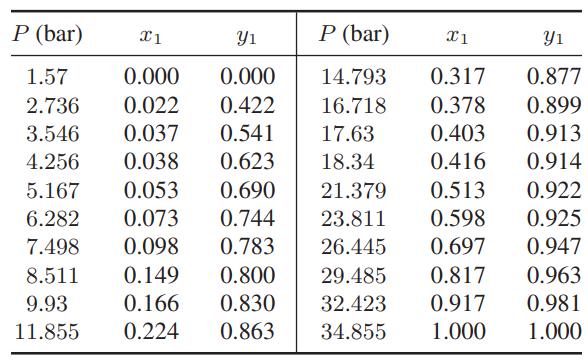
a. Find the value of the binary interaction parameter in the Peng-Robinson equation of state with the van der Waals one-fluid mixing rules that best fits these data, and plot the correlated results and experimental data on the same graph.
b. Find the partition coefficients Ki = yi/xi from both correlation and the experimental data for each species as a function of pressure, and plot all the partition coefficients as a function of pressure on a single graph.
P (bar) X1 Y 1.57 0.000 0.000 0.422 2.736 0.022 3.546 0.037 0.541 4.256 0.038 0.623 5.167 0.053 0.690 6.282 0.073 0.744 7.498 0.098 0.783 8.511 0.149 0.800 9.93 0.166 0.830 11.855 0.224 0.863 P (bar) x1 14.793 0.317 16.718 0.378 17.63 0.403 18.34 0.416 21.379 0.513 23.811 0.598 26.445 0.697 29.485 0.817 32.423 0.917 34.855 1.000 Y1 0.877 0.899 0.913 0.914 0.922 0.925 0.947 0.963 0.981 1.000
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


