Two streams containing pyridine and acetic acid at 25C are mixed and fed into a heat exchanger.
Question:
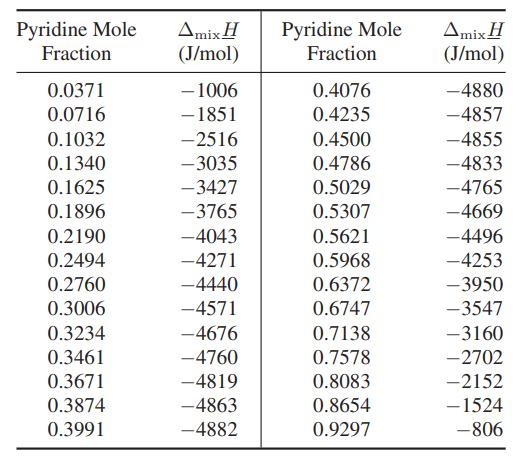
Two streams containing pyridine and acetic acid at 25°C are mixed and fed into a heat exchanger. Due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°C using a stream of chilled ethylene glycol as indicated in the diagram. Calculate the mass flow rate of ethylene glycol needed. The heat capacity of ethylene glycol at these conditions is approximately 2.8 kJ/(kg K), and the enthalpy change of mixing (ΔmixH) is given below.
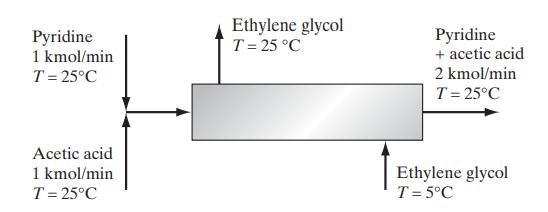
Data: Heat of mixing for pyridine (C5H5N) and acetic acid at 25°C [H. Kehlen, F. Herold and H.- J. Rademacher, Z. Phys. Chem. (Leipzig), 261, 809 (1980)].
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





