Question: Using the values for the equilibrium constant for the ionization of water in Table 13.5-1, estimate the standard-state heat of ionization of water as a
Using the values for the equilibrium constant for the ionization of water in Table 13.5-1, estimate the standard-state heat of ionization of water as a function of temperature. Also, determine the pH of water at each of the temperatures in this table.
Table 13.5-1
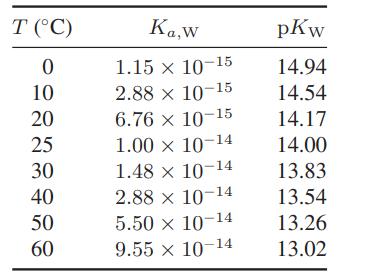
T (C) 0 10 20 25 30 40 50 60 Ka,w 1.15 x 10-15 2.88 x 10-15 6.76 10-15 1.00 x 10-14 1.48 10-14 x 2.88 x 10-14 5.50 x 10-14 9.55 x 10-14 pKw 14.94 14.54 14.17 14.00 13.83 13.54 13.26 13.02
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
To estimate the standardstate heat of ionization of water as a function of temperature using the values given for the equilibrium constant Kaw from th... View full answer

Get step-by-step solutions from verified subject matter experts


