When water and n-propanol are isothermally mixed, heat may be either absorbed (Q > 0) or evolved
Question:
When water and n-propanol are isothermally mixed, heat may be either absorbed (Q > 0) or evolved (Q
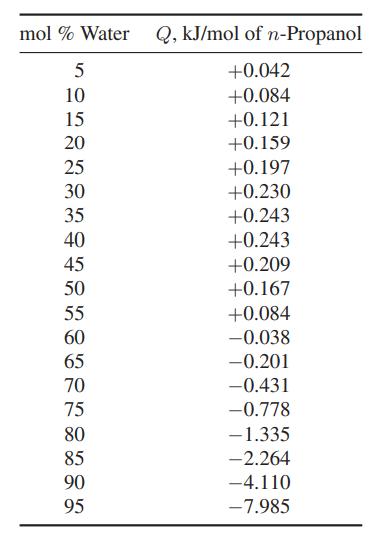
Plot (H̅W − HW) and (HNP − H̅NP) over the whole composition range.
Transcribed Image Text:
mol % Water Q, kJ/mol of n-Propanol 5 +0.042 10 +0.084 15 +0.121 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 +0.159 +0.197 +0.230 +0.243 +0.243 +0.209 +0.167 +0.084 -0.038 -0.201 -0.431 -0.778 -1.335 -2.264 -4.110 -7.985
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The molar integral heat of solution s H is defined as the change in enthalpy that results when 1 mole of solute (component 1) is isothermally mixed with N 2 moles of solvent (component 2) and is...
-
The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2) at 30C [reference: J. P. Shatas, M. M. Abbott, and H. C. Van Ness, J. Chem. Eng. Data, 20,...
-
Use the data in problem 8.29 to compute the partial molar enthalpies of pyridine and acetic acid in their mixtures at 25C over the whole composition range. Problem 8.29 Two streams containing...
-
The data in the chart below is for the distance (in cm) to the near point, the point nearest the eye at which the eye can accurately focus, at a persons age. Age (years) Near Point (cm) 10 7.5 20 9...
-
New West Fruit Corporation (New West) and Coastal Berry Corporation are both brokers of fresh strawberries. In the second half of 2009, New Wests predecessor, Moncs Consolidated Produce, Inc., loaned...
-
Create a SIPOC model for a project in which your university is modernizing its student center to include space for on-campus, student-run businesses. Be sure to include all relevant stakeholder...
-
5. What kinds of deductions are prohibited as a matter of public policy? Why might Congress deem it important to disallow deductions for expenditures that are against public policy?
-
Luis Corporation issued 1,000 shares of stock. Instructions Prepare the entry for the issuance under the following assumptions. (a) The stock had a par value of $5 per share and was issued for a...
-
Budget profit in foreign currency (FC) = 7,400; Actual profit in FC = 8,200 Exchange rate FC into U.S. dollars (USD): Beginning of year = 0.62; End of year = 0.69 (a) What is the total budget...
-
Design a clamper to perform the function indicated in Fig. 2.180. Silicon diodes 10 V 2.7 V vV Design -10 V
-
The heat-of-mixing data of Featherstone and Dickinson [J. Chem. Thermodyn., 9, 75 (1977)] for the n-octanol + n-decane liquid mixture at atmospheric pressure is approximately fit by with T in K and x...
-
Mattingley and Fenby [J. Chem. Thermodyn. 7, 307 (1975)] have reported that the enthalpies of triethylamine-benzene solutions at 298.15 K are given by where x B is the mole fraction of benzene and H...
-
Write a critically reflective paper entitled: My Role as a Decision Leader in the Global Marketplace. The purpose of this paper is for you to demonstrate your competency in discussing key concepts...
-
An increase in the price and a decrease of the quantity of Paclitaxel (an anti-cancer drug) could be caused by which of the following? Select one: O a. an increase in the number of people being...
-
At December 31, 2023, Cord Company's plant asset and accumulated depreciation and amortization accounts had balances as follows: Category Land Land improvements Buildings Equipment Automobiles and...
-
Assume that the following table represents the sales figures for the five largest firms in the industry. Compute the HHI for the industry (assuming the industry contains just these five firms). Sales...
-
Case study: Sun City - improving operations performance to enhance guest experience 1. Describe how Sun City implements the five operations performance objectives or principles. 2. Using your...
-
What recommendations do you have to increase the likelihood of success? E.g., how would you reduce the likelihood of having to go back to A4? How would you reduce the impact of having to go back to...
-
Determine vo and the required PIV rating of each diode for the configuration of Fig. 2.168. 100 V Ideal diodes -100 V 2.2 k
-
The cash records of Holly Company show the following four situations. 1. The June 30 bank reconciliation indicated that deposits in transit total $720. During July, the general ledger account Cash...
-
In 1925, the Try-State Tornado ripped a 219-mi path of destruction through Missouri, Illinois, and Indiana, killing a record 695 people. The maximum winds in the tornado were 318 mph. Express the...
-
Uphill water slides are becoming more popular at large water parks. Uphill speeds of riders can reach 19 ft/s. Express this speed in mph.
-
A brand-new engineering hire is late for her first product development team meeting. She gets out of her car and starts running 8 mph. It is exactly 7:58 a.m., and the meeting starts at exactly 8:00...
-
true- false statement (e) The objective of a family foundation is charity. (f) Limited liability offers bankruptcy protection to shareholders
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
You just won a stock picking contest that will pay you $24,000 a year for 26 years, and you get the first payment today. What is the prize worth to you today if your annual opportunity cost rate is...

Study smarter with the SolutionInn App


