(100.0 mathrm{kmol} / mathrm{h}) of a saturated vapor feed that is (25.0 mathrm{~mol} %) nitromethane (NM) and...
Question:
\(100.0 \mathrm{kmol} / \mathrm{h}\) of a saturated vapor feed that is \(25.0 \mathrm{~mol} \%\) nitromethane (NM) and \(75.0 \mathrm{~mol} \%\) water is separated in a system with two distillation columns and a liquid-liquid separator. The feed is sent to column \(\mathrm{W}\) that produces a water product that is \(1.0 \mathrm{~mol} \% \mathrm{NM}\). The boilup ratio in column \(\mathrm{W}\) is \(1 / 4\). Use the optimum feed stage. The vapor distillate from column \(\mathrm{W}\) is condensed and sent to the decanter. The water phase from the decanter \((8.6 \mathrm{~mol} \% \mathrm{NM})\) is refluxed to column W. The NM phase from the decanter ( \(31.2 \mathrm{~mol} \%\) water) is sent to stripping column NM. The nitromethane product from the bottom of stripping column NM is 2.0 \(\mathrm{mol} \%\) water. The boilup ratio in column \(\mathrm{NM}=3.0\). Assume both columns operate at \(1.0 \mathrm{~atm}\) pressure, \(\mathrm{CMO}\) is valid, both condensers are total condensers, and both reboilers are partial reboilers. Equilibrium data are in Table 8-8, Problem 8.D16. Density data are also given in Problem 8.D16. Be sure to read the note on safety at the end of Problem 8.D16.
Problem 8.D16.
Problem 8.D16.
Nitromethane and water are separated in a rectifying column system with a total condenser and a liquid-liquid settler similar to Figure 8-3A, except the column is a rectifying column. The saturated vapor feed is \(7.5 \mathrm{~mol} \%\) water. The water product from the settler is \(91.4 \mathrm{~mol} \%\) water. The organic phase refluxed to the column is \(31.2 \mathrm{~mol} \%\) water. Pressure \(=1.0 \mathrm{~atm}\), feed rate \(=50.0 \mathrm{kmol} / \mathrm{h}\), and \(\mathrm{CMO}\) is valid. The desired bottoms is 2.5 \(\mathrm{mol} \%\) water.
Figure 8-3A
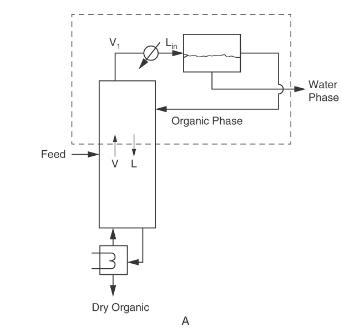
Find: \(\mathrm{x}_{\text {dist,water }}\), reflux ratio \(\mathrm{L} / \mathrm{D}, \mathrm{D}, \mathrm{B}\), and the number of equilibrium stages.
Equilibrium data are in Table 8-8. Density data are given after the table, and be sure to read the note on safety at the end of this problem.
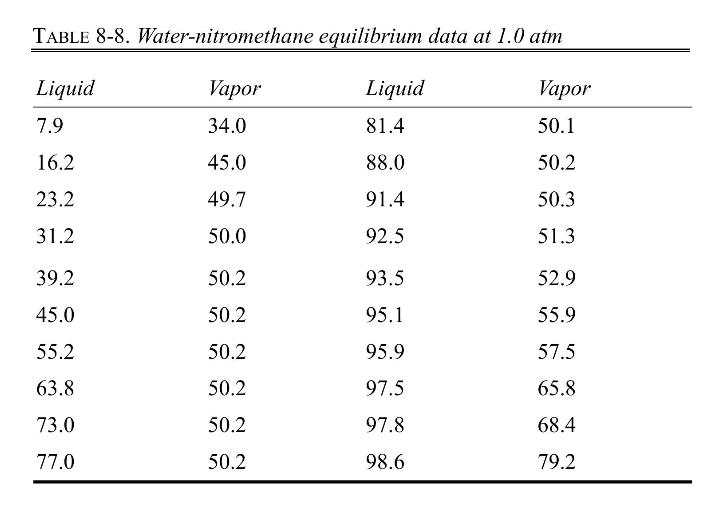
Data: Density of nitromethane at \(25^{\circ} \mathrm{C}\) is \(1129 \mathrm{~kg} / \mathrm{m}^{3}\).
Safety note: Nitromethane (also called methyl nitrate, \(\mathrm{CH}_{3} \mathrm{NO}_{2}\) ) is dangerous. If you try to freeze the pure compound, it explodes.
Find:
a. The flow rates of both products.
b. The optimum feed plate location and the number of stages in column W. Step off stages from the bottom up, and calculate a fractional number of stages.
c. The number of stages required in stripping column NM. Step off stages from the top down, and calculate a fractional number of stages.
d. The vapor flow rates entering each of the condensers.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





