A mixture of helium, oxygen, carbon dioxide, and nitrogen is fed to a perfectly mixed GP system
Question:
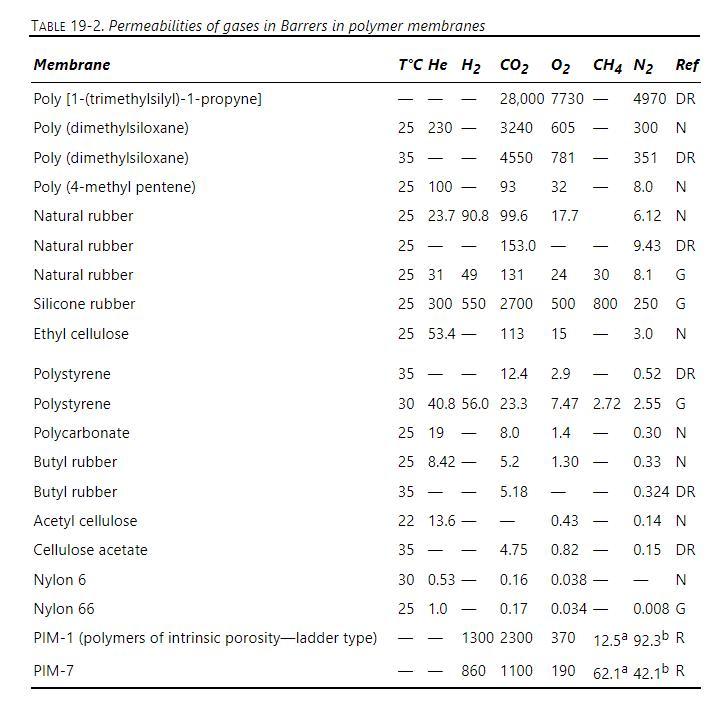
A mixture of helium, oxygen, carbon dioxide, and nitrogen is fed to a perfectly mixed GP system with a poly (dimethylsiloxane) membrane at \(25^{\circ} \mathrm{C}\). Membrane properties are given in Table 19-2. Membrane active layer thickness is \(1.2 \mu \mathrm{m}\). Feed pressure is \(6.4 \mathrm{~atm}=\) retentate pressure. Permeate pressure is \(1.03 \mathrm{~atm}\). Feed is 0.6 mole \% helium, \(57.5 \mathrm{~mole} \%\) carbon dioxide, \(13.5 \mathrm{~mol} \%\) oxygen, and 28.4 mole \% nitrogen. Feed rate is 1.13 \(\mathrm{m}^{3} / \mathrm{s}\) STP.
a.* Find retentate mole fractions, permeate mole fractions, and membrane area (in \(\mathrm{m}^{2}\) ) for \(\theta=0.36\).
b. Find retentate mole fractions, permeate mole fractions, and \(\theta\) for membrane area \(=100.0 \mathrm{~m}^{2}\).
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





