A nonvolatile solute is dissolved in (1.0 mathrm{kmol}) of methanol. We wish to have the solute in
Question:
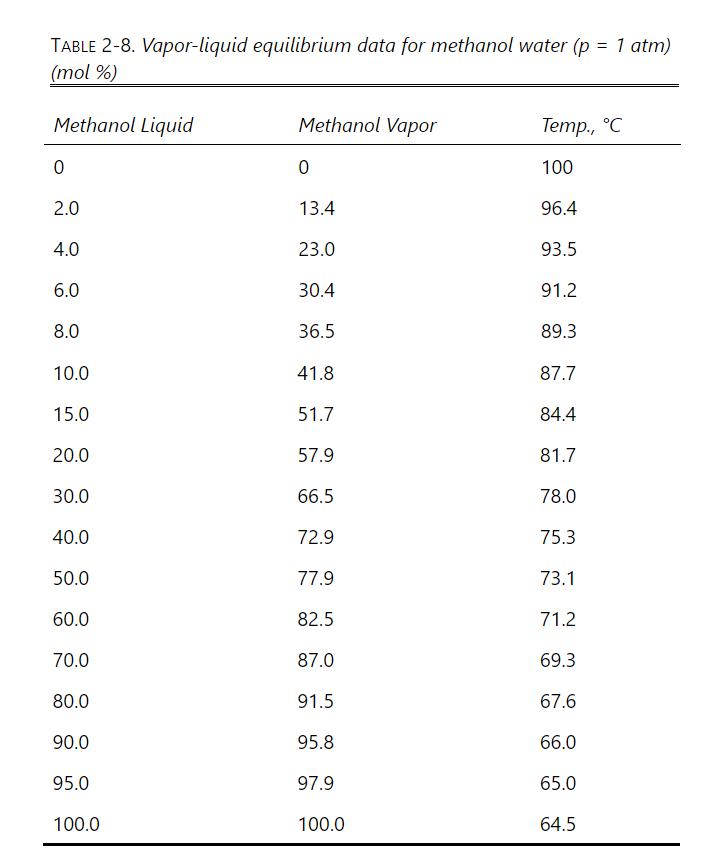
A nonvolatile solute is dissolved in \(1.0 \mathrm{kmol}\) of methanol. We wish to have the solute in \(1.0 \mathrm{kmol}\) of solution that is \(99.0 \mathrm{~mol} \%\) water and 1.0 \(\mathrm{mol} \%\) methanol. Because the solution is already concentrated, a first batch distillation to concentrate the solution is not required. VLE data (ignore the effect of the nonvolatile solute) are in Table 2-8. Do a constant-mole batch distillation from \(\mathrm{x}_{\mathrm{M}, \text { initial }}=1.0\) (pure methanol) to \(\mathrm{x}_{\mathrm{M}, \text { fin }}=0.01\). Find \(\mathrm{S}\), the moles of water evaporated with the methanol in the distillate, the kmol of distillate, and the methanol mole fraction in the distillate. Results can be compared with dilution followed by simple batch distillation in Problem 9.E4.
Table 2-8
Problem 9-E1

Because \(\mathrm{x}_{\mathrm{M}, \text { fin }}=0.01\) is quite small \((1 / \mathrm{y}=100\) at this limit), Simpson's rule is not accurate. Use the Gaussian quadrature formula instead.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





