Continuous steam distillation is used to recover 1 -octanol from (100.0 mathrm{~kg} / mathrm{h}) of feed that
Question:
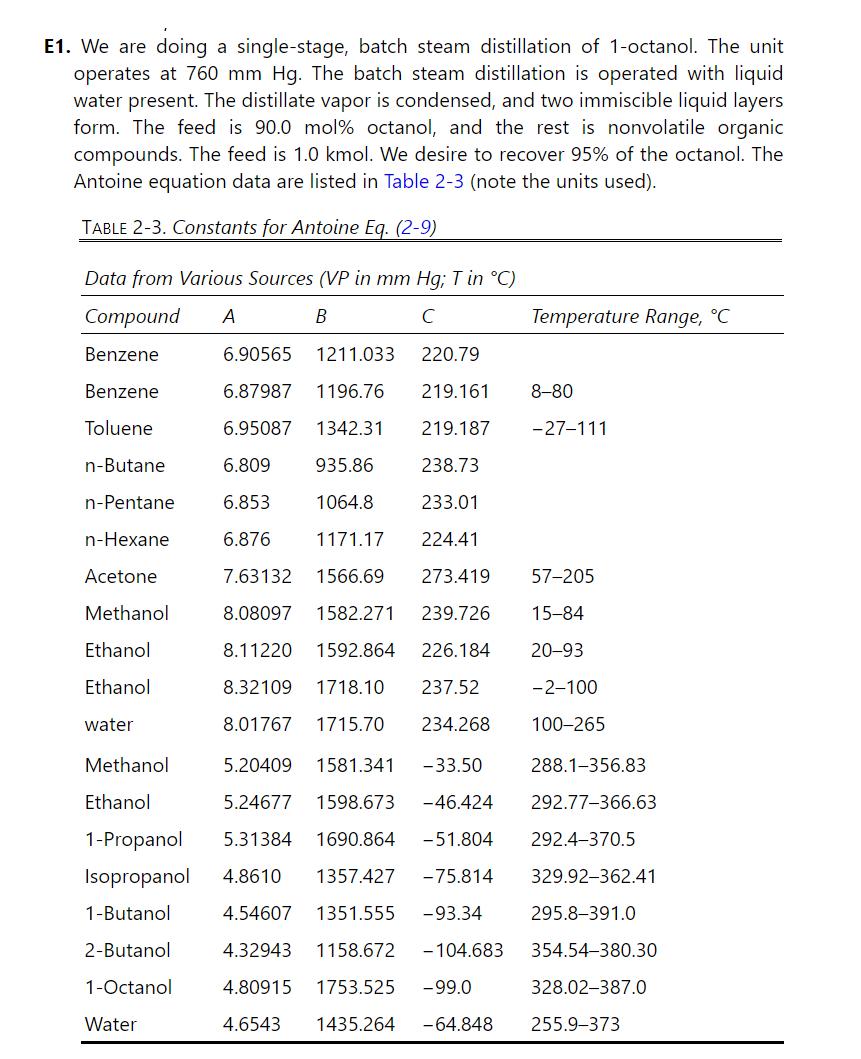
Continuous steam distillation is used to recover 1 -octanol from \(100.0 \mathrm{~kg} / \mathrm{h}\) of feed that is \(15.0 \mathrm{wt} \% 1\)-octanol, and the remainder consists of nonvolatile organics and solids of unknown composition. The feed is preheated to same temperature as the still pot, which operates at \(1.0 \mathrm{~atm}\) pressure. Operation is with liquid water in the pot. Assume the still pot is well mixed and the liquid and vapor are in equilibrium. Recover \(95.0 \%\) of 1 -octanol in distillate. Assume water is completely immiscible with \(1-\) octanol and with nonvolatile organics. Because the composition of the nonvolatile organics is not known, we boiled the feed mixture under a
vacuum with no water present. The result at \(0.05 \mathrm{~atm}\) pressure was the mixture boiled at \(129.8^{\circ} \mathrm{C}\).
a. Find the mole fraction of 1-octanol in the feed and the effective average molecular weight of the nonvolatile organics and solids.
b. Find the \(\mathrm{kg} / \mathrm{h}\) and \(\mathrm{kmol} / \mathrm{h}\) of \(1-\mathrm{octanol}\) in distillate, the \(\mathrm{kg} / \mathrm{h}\) of total organics in waste, and the 1-octanol weight fraction and the 1-octanol mole fraction in the waste.
c. Find the temperature of the still pot. A spreadsheet or MATLAB is highly recommended.
d. Find the \(\mathrm{kg} / \mathrm{h}\) and \(\mathrm{kmol} / \mathrm{h}\) of water in distillate.
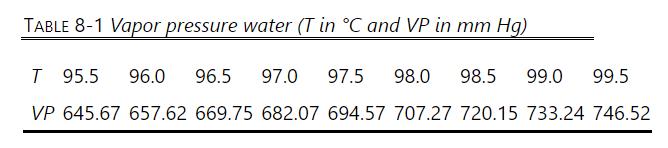
Octanol boils at about \(195^{\circ} \mathrm{C}\). The formula for octanol is \(\mathrm{CH}_{3}\left(\mathrm{CH}_{2}\right)_{6} \mathrm{CH}_{2} \mathrm{OH}\), and its molecular weight is 130.23 . Antoine coefficients for octanol and water are available in Table 2-3, and a table of water vapor pressure is in Table 8-1.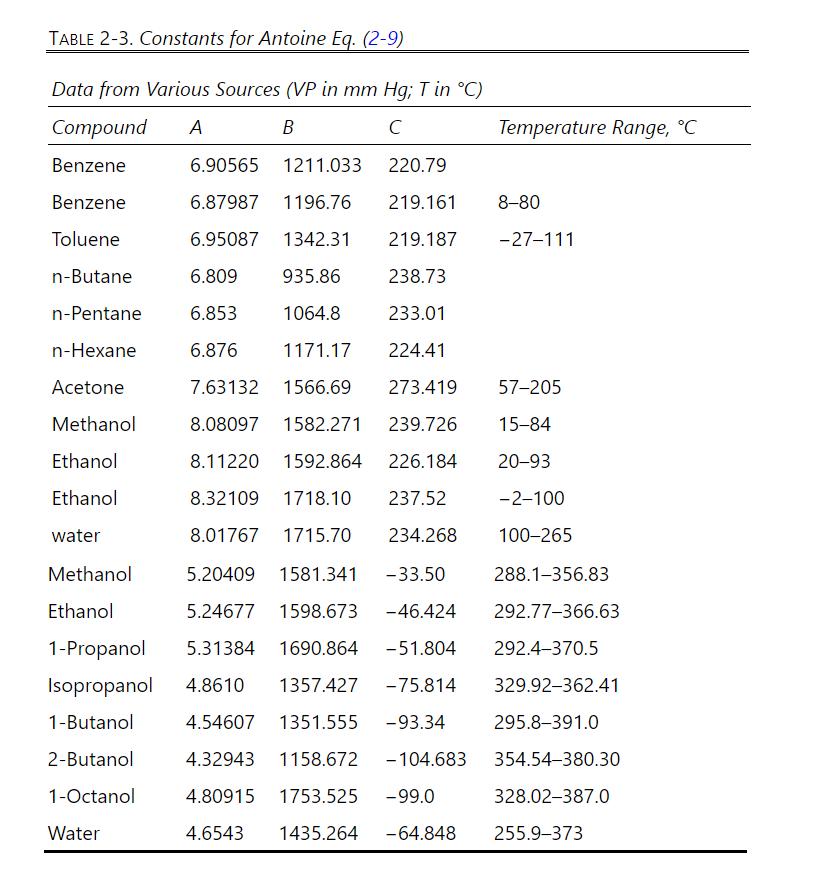
 We will see in Problem 9.E1 that batch steam distillation requires less steam than single-stage continuous steam distillation.
We will see in Problem 9.E1 that batch steam distillation requires less steam than single-stage continuous steam distillation.
Problem 9.E1
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





