What is the dew point of a vapor that is (30.0 mathrm{~mol} % mathrm{n})-butane, (50.0 mathrm{~mol} %
Question:
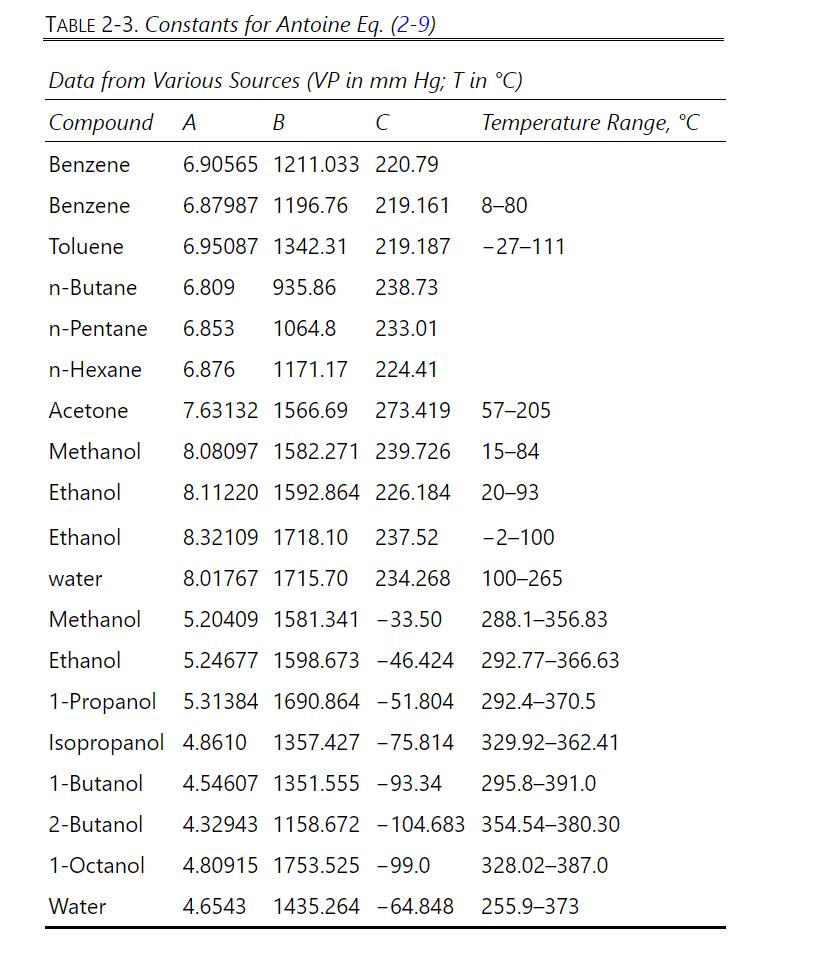
What is the dew point of a vapor that is \(30.0 \mathrm{~mol} \% \mathrm{n}\)-butane, \(50.0 \mathrm{~mol} \% \mathrm{n}\) pentane, and \(20.0 \mathrm{~mol} \% \mathrm{n}\)-hexane at \(\mathrm{p}=760.0 \mathrm{~mm} \mathrm{Hg}\) ? Use Raoult's law to predict K values. Find vapor pressures from Antoine's Eq. (2-11) and Antoine constants from Table 2-3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





