A vapor at (1.0 mathrm{~atm}) that is (60 mathrm{~mol} %) acetone and (40 mathrm{~mol} %) ethanol is
Question:
A vapor at \(1.0 \mathrm{~atm}\) that is \(60 \mathrm{~mol} \%\) acetone and \(40 \mathrm{~mol} \%\) ethanol is cooled until the first drop of liquid condenses. What is the mol fraction of the first drop of liquid, and what is the temperature? Be as accurate as you can. Equilibrium data are in problem 4.D7.
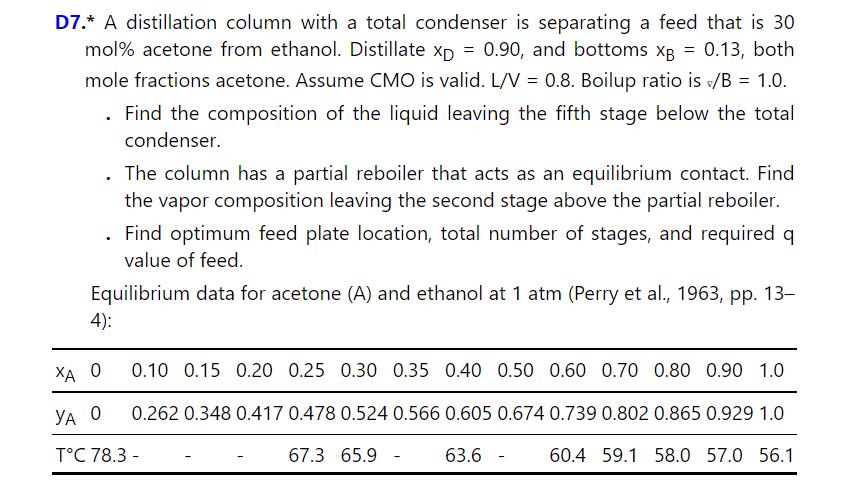
Problem 4.D7.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





