Question: For the reactor-distillation process in Example 22.5, recompute the solution when the reactor and column volumes are decreased by (20 %). Data From Example 22.5:-
For the reactor-distillation process in Example 22.5, recompute the solution when the reactor and column volumes are decreased by \(20 \%\).
Data From Example 22.5:-
Equation 22.9 and 22.11:-


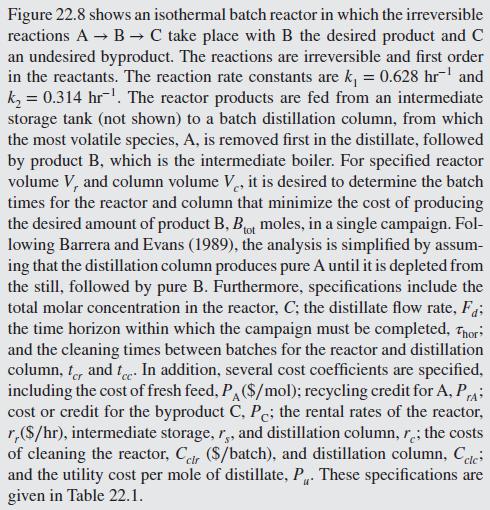
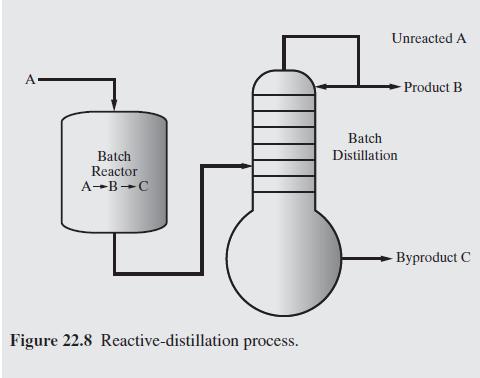
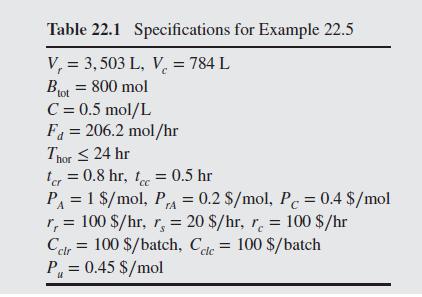
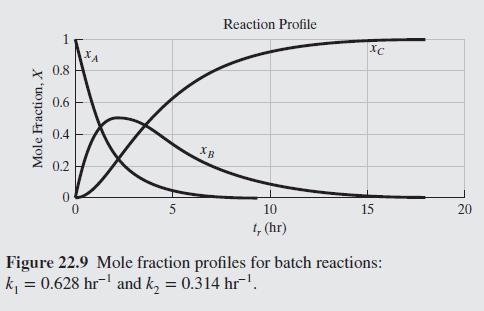
Figure 22.8 shows an isothermal batch reactor in which the irreversible reactions A B C take place with B the desired product and C an undesired byproduct. The reactions are irreversible and first order in the reactants. The reaction rate constants are k = 0.628 hr and k = 0.314 hr. The reactor products are fed from an intermediate storage tank (not shown) to a batch distillation column, from which the most volatile species, A, is removed first in the distillate, followed by product B, which is the intermediate boiler. For specified reactor volume V, and column volume V, it is desired to determine the batch times for the reactor and column that minimize the cost of producing the desired amount of product B, Bot moles, in a single campaign. Fol- lowing Barrera and Evans (1989), the analysis is simplified by assum- ing that the distillation column produces pure A until it is depleted from the still, followed by pure B. Furthermore, specifications include the total molar concentration in the reactor, C; the distillate flow rate, Fd; the time horizon within which the campaign must be completed, Thor and the cleaning times between batches for the reactor and distillation column, and t In addition, several cost coefficients are specified, including the cost of fresh feed, PA ($/mol); recycling credit for A, P cost or credit for the byproduct C, Pc; the rental rates of the reactor, r,($/hr), intermediate storage, r,, and distillation column, r; the costs of cleaning the reactor, Cir (S/batch), and distillation column, Cele and the utility cost per mole of distillate, P. These specifications are given in Table 22.1. PrA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


