a. Carbonate buffers are important in regulating the (mathrm{pH}) of blood at 7.40. What is the concentration
Question:
a. Carbonate buffers are important in regulating the \(\mathrm{pH}\) of blood at 7.40. What is the concentration ratio of \(\mathrm{CO}_{2}\) (usually written \(\mathrm{H}_{2} \mathrm{CO}_{3}\) ) to \(\mathrm{HCO}_{3}{ }^{-}\)in blood at \(\mathrm{pH}=7.40\) ?
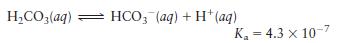
b. Phosphate buffers are important in regulating the \(\mathrm{pH}\) of intracellular fluids at \(\mathrm{pH}\) values generally between 7.1 and 7.2. What is the concentration ratio of \(\mathrm{H}_{2} \mathrm{PO}_{4}{ }^{-}\)to \(\mathrm{HPO}_{4}{ }^{2-}\) in intracellular fluid at \(\mathrm{pH}=7.15\) ?
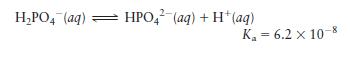
c. Why is a buffer composed of \(\mathrm{H}_{3} \mathrm{PO}_{4}\) and \(\mathrm{H}_{2} \mathrm{PO}_{4}{ }^{-}\) ineffective in buffering the \(\mathrm{pH}\) of intracellular fluid?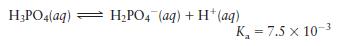
Step by Step Answer:






