A typical bathtub can hold 100. gallons of water. (a) Calculate the mass of natural gas that
Question:
A typical bathtub can hold 100. gallons of water.
(a) Calculate the mass of natural gas that would need to be burned to heat the water for a tub of this size from 65 °F to 108 °F. Assume that the natural gas is pure methane, CH4.
(b) What volume of natural gas does this correspond to at 25 °C and 1.00 atm? See Table 4D.1.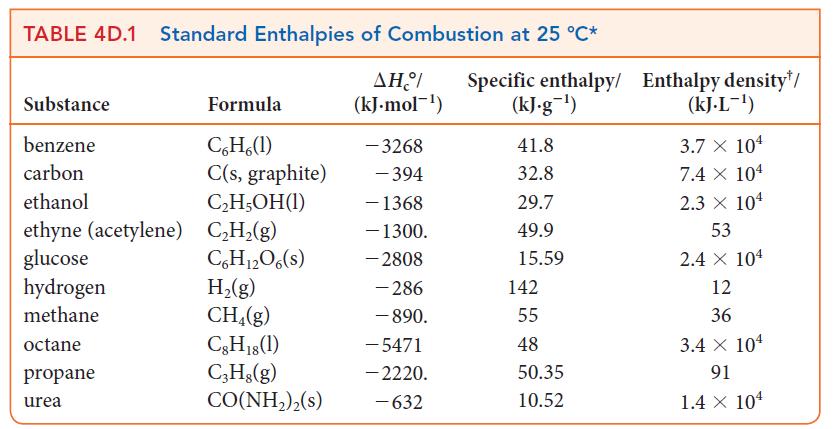
Transcribed Image Text:
TABLE 4D.1 Standard Enthalpies of Combustion at 25 °C* Substance benzene carbon ethanol ethyne (acetylene) glucose hydrogen methane. octane propane urea Formula C6H6(1) C(s, graphite) C₂H5OH(1) C₂H₂(g) С6H12O6(s) H₂(g) CH₂(g) C8H18 (1) C3H8(g) CO(NH2)2(s) ΔΗ/ (kJ.mol-¹) -3268 - 394 - 1368 - 1300. - 2808 - 286 - 890. -5471 - 2220. -632 Specific enthalpy/ Enthalpy density*/ (kJ.g-¹) 41.8 32.8 29.7 49.9 15.59 142 55 48 50.35 10.52 (kJ.L-¹) 3.7 X 104 7.4 X 104 2.3 × 104 53 2.4 X 104 12 36 3.4 x 104 91 1.4 X 104
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The mass of natural gas that would need to be burned to heat the water for a tub of this size from 6...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A five-year follow-up study was carried out in a certain metropolitan area to assess the relationship of diet and weight to the incidence of stomach cancer. Data were obtained on n = 2,000 subjects....
-
Adelyn is in a financial dispute with her creditor. She wants to declare bankruptcy because she is finding herself unable to meet the requirements of paying off her debt. Which court that A would...
-
The following is the current balance sheet for a local partnership of doctors: Cash and current assets Land Building and equipment (net) $ 30,000 150,000 140,000 Liabilities A, capital B, capital $...
-
A Xerox machine in a shop is operated by a person who does other jobs too. The average service time for a job is 6 minutes per customer. On an average, in every 12 minutes one customer arrives for...
-
An analysis of the general ledger accounts indicates that office equipment, which cost $67,000 and on which accumulated depreciation totaled $22,500 on the date of sale, was sold for $38,600 during...
-
Refer to the Journal of Biogeography (Dec. 2003) study of ants in Mongolia (Central Asia), Exercise 8.98, where you compared the mean number of ants at two desert sites. Since the sample sizes were...
-
2-11. How do the goals set for a marketing program in the planning phase relate to the evaluation phase of the strategic marketing process?
-
An office employs several clerks who create documents and one operator who enters the document information in a computer system. The group creates documents at a rate of 25 per hour. The operator can...
-
On December 31, 2024, Tamarisk Corporation signed a 5-year, non-cancelable lease for a machine. The terms of the lease called for Tamarisk to make annual payments of $7,880 at the beginning of each...
-
Benzoic acid has a known internal energy of combustion (3251 kJ mol 1 ). When a bomb calorimeter was calibrated by burning 0.825 g of benzoic acid in oxygen, the temperature rose 1.94C. A sample of...
-
Calculate the change in molar Gibbs free energy for the process CH 4 (l) CH 4 (g) at 1 atm and (a) 140.0C; (b) 180.C. In each case, indicate whether or not vaporization would be spontaneous.
-
Record adjustments. (LO 1, 2, 3) The following is a list of financial statement items from Chunky Candy Company as of June 30, 2008. Chunky's fiscal year is from July 1 to June 30. Additional...
-
Peninsula Community Health Services of Alaska had just completed of a merger of two organizations. The original Peninsula Community Health center was a community health center only, but the CHC had...
-
Compensation Approach: Imagine that the HR department of your chosen organization from below is going to design a compensation approach for the job that is aligned with reinforcement, expectancy, and...
-
A boat leaves port and follows a course of N77E at 9 knots for 3 hr and 20 min. Then, the boat changes to a new course of S26E at 12 knots for 5 hr. Part 1 of 3 (a) How far is the boat from port?...
-
The aggregate supply curve of an economy is depicted by AS, shown in the graph on the right. Suppose that labour unions grant concessions, enabling firms to pay lower wages to their workers. Use the...
-
what is Medibank pestle analysis in term of these 2 statements? Current problem at hand deviates towards the fact that customers do not have high awareness of the health and wellbeing programs that...
-
Draw qualitative diagrams for the crystal-field splitting in (a) A linear complex ion ML2, (b) A trigonalplanar complex ion ML3, and (c) A trigonal-bipyramidal complex ion ML5.
-
Propose a reasonable mechanism for the following reaction. OH
-
How many atoms of nitrogen are present in \(5.00 \mathrm{~g}\) of each of the following? a. Glycine, \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{O}_{2} \mathrm{~N}\) b. Magnesium nitride c. Calcium...
-
Consider the equation \(3 \mathrm{~A}+\mathrm{B} ightarrow \mathrm{C}+\mathrm{D}\). You react 4 moles of A with 2 moles of \(\mathrm{B}\). Which of the following is true? a. The limiting reactant is...
-
Atoms of three different elements are represented by \(\mathrm{O}\), \(\square\), and \(\Delta\). Which compound is left over when three molecules of \(\mathrm{O} \Delta\) and three molecules of...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


