Balance the following equations using the smallest whole number coefficients, then write the expression for K for
Question:
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: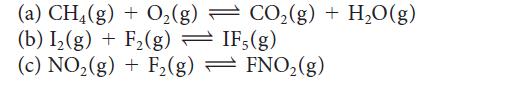
Transcribed Image Text:
(a) CH4(g) + O₂(g) (b) I₂(g) + F₂(g) → IF, (g) (c) NO₂(g) + F₂(g) = FNO₂(g) CO₂(g) + H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a CH4g 2Og COg 2 H0g ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: (a) CH4(g) + Cl(g) CHCl(g) + HCl(g) (b) NH3(g) + ClF3(g) (c) N(g) +...
-
1. Given Pascal's Triangle 4 1 3 1 2 6 1 3 1 5 10 10 4 1 5 1 1 a. Explain how the n and r values for C, relate to the number 6 shown above in Pascal's triangle. Justify your explanation by showing...
-
Balance the following equations using the method outlined in Section 3.7: (a) C + O2 CO (b) CO + O2 CO2 (c) C1 + Br2 HBr (d) K + C1O KOH + C1 (e) Mg + O2 MgO (f) O3 O2 (g) C1O2 C1O + O2 (h) N2 + C1...
-
Suppose that today, you paid $1,000 for the bond described in Problem 8 of Chapter 8, The net present value functions: NPV and XNPV. What would be the bonds IRR? A bonds IRR is often called the yield...
-
Isopropanol, containing 13 wt% water, can be dehydrated to obtain almost pure isopropanol at a 90% recovery by azeotropic distillation with benzene. When condensed, the overhead vapor from the column...
-
A geared industrial roll shown in the figure is driven at 300 rev/min by a force F acting on a 3-in-diameter pitch circle as shown. The roll exerts a normal force of 30 lbf/in of roll length on the...
-
Use a worksheet for employers pension plan entries.
-
Alec, Daniel, William, and Stephen decide today to save for retirement. Each person wants to retire by age 65 and puts $11,000 into an account earning 10% compounded annually. Required: Calculate how...
-
Examine the Dilemmas on HO 5 and state how you would deal with them based on your knowledge of the Conduct of Business Regulations.
-
1. If you were in Jimmies shoes, would you sell Greg an equity stake in Lees Ice Cream? Explain. If Jimmie does sell equity to Greg for $3,300, what percentage of the business should he offer? 2....
-
You have two beakers: one is filled with tetrachloromethane and the other with water. You also have two compounds, butane(CH 3 CH 2 CH 2 CH 3 ) and calcium chloride. (a) In which liquid will butane...
-
A reactor for the production of ammonia by the Haber process is found to be at equilibrium with P N2 = 3.11 bar, P H2 = 1.64 bar, and P NH3 = 23.72 bar. If the partial pressure of N 2 is increased by...
-
For cold-worked copper, construct a plot for the time to 50% recrystallization as a function of annealing temperature.
-
Childhood leukemia, a hematological malignancy, is the most common form of childhood cancer, representing 29% of cancers in children aged 0 to 14 years in 2018. Imagine that you work in the State...
-
Question 1 Approximating functions using linear functions or higher degree polynomials is a very useful scientific tool! This concept generalizes to Taylor Polynomials, but is most simply illustrated...
-
Find the volume of the solid of revolution formed by rotating the specified region R about the x axis. Volume Formula Suppose f(x) is continuous and f(x) 0 on a x b, and let R be the region under the...
-
As machines get older, the cost of maintaining them tends to increase. Suppose for a particular machine, the rate at which the maintenance cost is increasing is approximated by the function C' (t) =...
-
At Edsel Automotive, the management team is planning to expand one of its plants by adding a new assembly line for sport utility vehicles (SUVs). The cost of setting up the new SUV assembly line is...
-
(a) What architecture do most firms actually use? (b) In the hybrid TCP/IP-OSI architecture, which layers come from OSI? (d) From what standards architecture do application layer standards come?
-
General Electric Capital, a division of General Electric, uses long-term debt extensively. In a recent year, GE Capital issued $11 billion in long-term debt to investors, then within days filed legal...
-
Compare the structure of vitamin E with the structures of BHT and BHA, and then determine which hydrogen atom is most easily abstracted from vitamin E.
-
Using your results from Problems P5.18 and P5.7, calculate ÎS, ÎS surroundings , and ÎS total for each step in the cycle and for the total Carnot cycle described in Figure 5.2....
-
Predict the products for each reaction. In each case, be sure to consider whether a chirality center is being generated and then draw all expected stereoisomers. (a) (b) (c) (d) (e) (f) H ROOR HBr...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


