Balance the following equations using the smallest whole number coefficients, then write the expression for K for
Question:
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: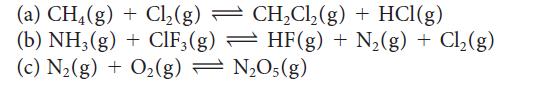
Transcribed Image Text:
(a) CH4(g) + Cl₂(g) — CH₂Cl₂(g) + HCl(g) (b) NH3(g) + ClF3(g) (c) N₂(g) + O₂(g)= HF (g) + N₂(g) + Cl₂(g) N₂O5(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Lets balance the chemical equations and write the ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations using the smallest whole number coefficients, then write the expression for K for each reaction: (a) CH4(g) + O(g) (b) I(g) + F(g) IF, (g) (c) NO(g) + F(g) = FNO(g)...
-
1. Given Pascal's Triangle 4 1 3 1 2 6 1 3 1 5 10 10 4 1 5 1 1 a. Explain how the n and r values for C, relate to the number 6 shown above in Pascal's triangle. Justify your explanation by showing...
-
Balance the following equations using the method outlined in Section 3.7: (a) C + O2 CO (b) CO + O2 CO2 (c) C1 + Br2 HBr (d) K + C1O KOH + C1 (e) Mg + O2 MgO (f) O3 O2 (g) C1O2 C1O + O2 (h) N2 + C1...
-
In Problems 2628, find the value of each determinant. 21 5 0 26 -3 1 0
-
For the binary system ethanol(l)/isooctane(2) at 50?C, the infinite-dilution, liquid-phase activity coefficients are ?1? = 21.17 and ?2? = 9.84.(a) Calculate the constants A12 and All in the van Laar...
-
A 50.0-g copper calorimeter contains 250 g of water at 20.0C. How much steam must be condensed into the water if the final temperature of the system is to reach 50.0C?
-
(Basic Pension Worksheet) The following facts apply to the pension plan of Trudy Borke Inc. for the year 2011. Plan assets, January 1, 2011 $490,000 Projected benefit obligation, January 1, 2011...
-
A tanning parlor located in a major shopping center near a large New England city has the following history of customers over the last four years (data are in hundreds of customers and months are the...
-
A petty cash fund was originally established with a check for $100. On August 31, which is the period end, the petty cash fund included the following: PETTY CASH RECEIPTS Postage Office Supplies...
-
Use Nodal Analysis to find Vx, in the circuit. 202 4A -2 A Va VB 50 N 40 2 100 2 25 2 10 A
-
The normal boiling point of ethanol is 78.4 C. When 9.15 g of a soluble nonelectrolyte was dissolved in 100. g of ethanol, the vapor pressure of the solution at that temperature was 7.40 * 10 2 Torr....
-
A 0.124 m CCl 3 COOH(aq) solution has a freezing point of 20.423C. What is the percentage deprotonation of the acid?
-
In Section 7.5, we discllssed nonpreemptive priority queuing. What would be preemptive pliority queuing? Does preemptive priority queuing make sense for computer networks?
-
Joint Ventures are a common Mode of Entry in international business. Appreciate if in-depth elaboration provided on its advantages and disadvantages. Also briefly mention the factors which make joint...
-
The field excursion is intended to give students an opportunity to carry out an applied geographical research project based on observation, data recording, and analysis. Using a field site of your...
-
An angry coworker is expressing their needs through a rush of emotion and snide comments while another coworker is trying to interpret them to provide some help and support. You are a manager and...
-
You may have a general understanding of the difference between ethics and legality , but could you explain the distinction? It is not always easy to know where to draw the line between the two. Some...
-
Someone can be a good leader but not be a very good manager and vice-versa. Leadership is creating a vision for others to follow, establishing corporate values and ethics, and transforming the way...
-
(a) What is the TCP/IP internet layer supervisory protocol? (b) Describe ping. (c) Describe ICMP error messages. (d) What information does ping give an attacker? (e) What information does tracert...
-
In a nonmagnetic medium, E = 50 cos (10 9 t 8x) a y + 40 sin (10 9 t 8x) a z V/m find the dielectric constant r and the corresponding H.
-
Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using the RedlichKwong equation of state: The RedlichKwong parameters a and b for benzene are 452.0 bar dm 6 mol 2 K...
-
The triphenylmethyl radical was the first radical to be observed. Draw all resonance structures of this radical, and explain why this radical is unusually stable:
-
Use the equation C P,m C V ,m = TV m 2 / and the Data Tables to determine C V ,m for H 2 O(l) at 298 K. Calculate (C p,m C V,m )/C P,m .
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...
-
Only need help on 4B and 5. Exercise 9-21 Breakeven Planning; Profit Planning (LO 9-2, 9-3] Connelly Inc., a manufacturer of quality electric ice cream makers, has experienced a steady growth in...

Study smarter with the SolutionInn App


