Below is the titration curve for the neutralization of 25 mL of a base with a strong
Question:
Below is the titration curve for the neutralization of 25 mL of a base with a strong monoprotic acid. Answer the following questions about the reaction and explain your reasoning in each case.
(a) Is the base strong or weak?
(b) What is the initial hydroxide ion concentration of the base?
(c) What is Kb for the base?
(d) What is the initial concentration of the base?
(e) What is the concentration of acid in the titrant?
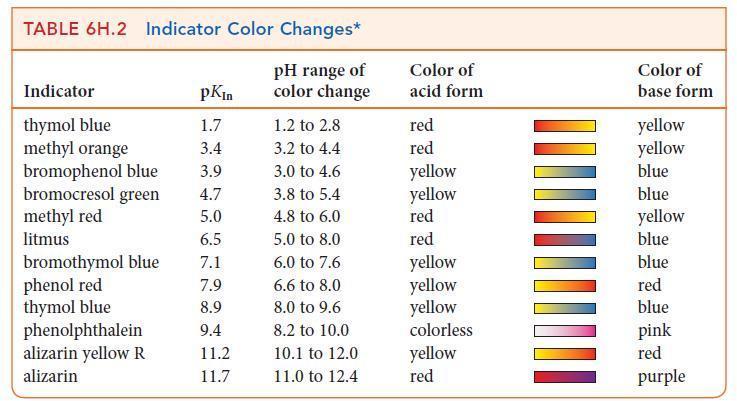
(f) Use Table 6H.2 to select an indicator for the titration.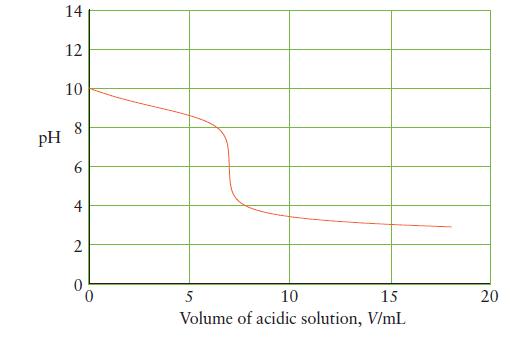
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





