Benzene is more stable and less reactive than would be predicted from its Kekul structures. Use the
Question:
Benzene is more stable and less reactive than would be predicted from its Kekulé structures. Use the data in Table 4E.3 to calculate the lowering in molar energy when resonance is allowed between the Kekulé structures of benzene.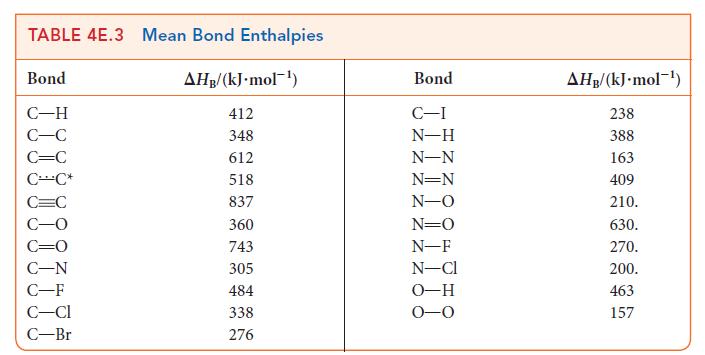
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





