Butadiene reacts to form its dimer according to the equation 2C 4 H 6 (g) C
Question:
Butadiene reacts to form its dimer according to the equation 2C4H6(g) → C8H12(g) The following data were collected for this reaction at a given temperature:
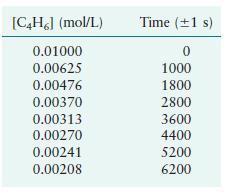
a. Is this reaction first order or second order?
b. What is the value of the rate constant for the reaction?
c. What is the half-life for the reaction under the conditions of this experiment?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





