The balanced equation for the reaction of gaseous nitrogen dioxide and fluorine is The experimentally determined rate
Question:
The balanced equation for the reaction of gaseous nitrogen dioxide and
fluorine is
![]()
The experimentally determined rate law is
![]()
A suggested mechanism for this reaction is
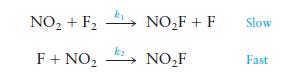
Is this an acceptable mechanism? That is, does it satisfy the two requirements?
Transcribed Image Text:
2NO(g) + F(g) 2NOF(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The first requirement for an acceptable mechanism is that the sum of ...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
3. Calculate and Explain, how much the action described has added to GDP: A company sells 50 computers at a retail price of $1000 apiece and 100 software packages at a retail price of $50 apiece to...
-
The reaction of nitric oxide (NO(g)) with molecular hydrogen (H2(g)) results in the production of molecular nitrogen and water as follows: 2NO(g) + 2H 2 (g) N 2 O(g) + 2H 2 O(g) The experimentally...
-
The dissolved oxygen present in any highly pressurized, hightemperature steam boiler can be extremely corrosive to its metal parts. Hydrazine, which is completely miscible with water, can be added to...
-
Here is the end-of-year account balance information from the accounting records of Jaunty Coffee Company: Sales revenue Cash Cost of goods sold Accounts payable Capital stock Dividends Retained...
-
(a) Use a computer algebra system to draw a direction field for the differential equation. Get a printout and use it to sketch some solution curves without solving the differential equation. (b)...
-
What was the scale of this change for those staff involved in the process, that is, those who were expected to become generic workers?
-
Suppose you fit a least squares line to 12 data points and the calculated value of SSE is .429. a. Find s2, the estimator of s2 (the variance of the random LO9 error term e). b. Find s, the estimate...
-
At the end of Hotai Department Stores fiscal year on December 31, 2020, these accounts appeared in its adjusted trial balance. Freight-In ..........................................................NT$...
-
I have seen the same question but for mine the production quantity where the annual worth of the two machines is the same at an interest rate of 10%! Thank you :) salvage value: 80,000 Annual Op...
-
The gas-phase reaction of chlorine with chloroform is described by the equation The rate law determined from experiment has a noninteger order: A proposed mechanism for this reaction follows: Is this...
-
Butadiene reacts to form its dimer according to the equation 2C 4 H 6 (g) C 8 H 12 (g) The following data were collected for this reaction at a given temperature: a. Is this reaction first order or...
-
Martin Realtors, a real estate consulting firm, specializes in advising companies on potential new plant sites. The company uses a job order costing system with a predetermined indirect cost...
-
Question: Divide.21r7-35r37r3r8-5r43r7-5r33r6-5r221r6-35r2 Divide. 2 1 r7 - 3 5 r3 7r 3r 8 - 5 r4 3 r7 - 5 r3 3 r6 - 5 r2 2 1 r6 - 3 5 r2 Divide. 21r7 - 35r 7r 38-54 3r7-53 3r6-52 216-3512
-
System of Equations: Value of a Value of b 9 a + 3 b = 3 0 8 a + 4 b = 2 8
-
An important practice is to check the validity of any data set that you analyze. One goal is to detect typos in the data, and another would be to detect faulty measurements. Recall that outliers are...
-
ve The re e Problem 3 Complete the following perpetual inventory form. Perpetual Inventory Total Product Name: Purchase Unit Size: Carried Forward: Date In Out 1/7 3 Balance 1/9 1/10 1/12 1/15 2 1 5...
-
the above date, Saloni was admitted in the partnership firm. Raman surrendered th 5 2 of his share and Rohit surrendered th 5 1 of his share in favour of Saloni. It was agreed that : (i) Plant and...
-
The model in Section 5.5, Exercise 40. Find the null clines and equilibria of this model when = 2.0, = 1.0, and b = 1.0. Draw the null clines and find equilibria of the above extensions of the...
-
The National Collegiate Athletic Association (NCAA) and the National Federation of State High School Associations (NFHS) set a new standard for non-wood baseball bats. Their goal was to ensure that...
-
Draw Lewis structures for each of the following species and predict the hybridization at each carbon atom: (a) H 2 CCH ; (b) H 2 CCH 3 + ; (c) H 3 CCH 2 .
-
There are three isomers of difluoroethene, C 2 H 2 F 2 , which differ in the locations of the fluorine atoms. (a) Which of the forms are polar? (b) Which has the largest dipole moment? F F C= 1 C O H...
-
Explain why the lattice energy of lithium chloride (861 kJ mol -1 ) is greater than that of rubidium chloride (695 kJ mol -1 ), given that they have similar arrangements of ions in the crystal...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


